Sky Medical Technology recently announced US Food and Drug Administration (FDA) 510(k) clearance to market the Geko device for increasing microcirculatory blood flow in lower limb soft tissue of patients with venous insufficiency and/or ischaemia.
According to Sky Medical, the Geko device is the first electrical neuromuscular stimulator of its kind to be cleared for increasing microcirculatory blood flow and adds to Sky’s prior FDA clearances for oedema reduction and for stimulation of the calf muscles to prevent venous thrombosis in both surgical and non-surgical patients.
Venous insufficiency can progress to chronic venous insufficiency (CVI), a serious condition attributed to diminished quality of life and loss of work productivity. In most cases, the cause is incompetent valves. Each year approximately 150,000 new patients are diagnosed with chronic venous insufficiency, and nearly US$500 million is used in the care of these patients, the company states. If venous insufficiency and ischaemia are left untreated, progressive to CVI can lead to post-phlebitic syndrome and venous leg ulcers.
CEO and founder Bernard Ross said, “Achieving this latest 510(k) clearance is a significant milestone for Sky that will allow us to initiate a controlled market release of the Geko device, to address venous insufficiency and ischaemia in the first instance—a therapy area sorely in need of innovation. With this 510(k) and in partnership with leading US clinicians we can now press ahead to redefine the way vascular related conditions can be treated.”
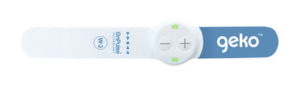
The Geko device is a non-invasive, easy-to-use, wearable therapy device, a press release details. The size of a wristwatch and worn at the knee, the disposable device gently stimulates the common peroneal nerve activating the calf and foot muscle pumps, resulting in increased blood flow in the deep veins of the calf, at a rate equal to 60% of walking without a patient having to move. The device operates without external pressure to the leg and allows complete mobility.












