Tag: thrombectomy
Enrolment in SPIRARE II pivotal trial of embolectomy system for pulmonary...
Jupiter Endovascular today announced completion of patient enrolment in the SPIRARE II pivotal trial evaluating the Vertex pulmonary embolectomy system in patients with acute,...
SonoVascular completes first close of Series A preferred stock financing
SonoVascular, developer of the SonoThrombectomy system for the treatment of venous thromboembolism (VTE), has completed the first close of its US$6 million Series A...
Boston Scientific announces agreement to acquire Penumbra
Boston Scientific and Penumbra today announced the companies have entered into a definitive agreement under which Boston Scientific will acquire Penumbra in a cash...
Medtronic announces first commercial use of Liberant thrombectomy system
Medtronic has announced the first commercial use of its Liberant thrombectomy system, which is indicated for the removal of fresh, soft emboli or thrombi...
Penumbra receives CE mark for Lightning Bolt 12 and Lightning Bolt...
Penumbra recently announced that it has secured CE-mark approval for the Lightning Bolt 12 and Lightning Bolt 6X with TraX, which the company notes...
New Medicare analysis shows increased thrombectomy use in venous disease
A new review of Medicare data shows a marked increase for both arterial and venous thrombectomy claims for venous thromboembolic disease filed between 2017–2022.
Researchers...
InterVene receives US FDA 510(k) clearance for Recana thrombectomy system
InterVene recently announced it has received 510(k) clearance from the US Food and Drug Administration (FDA) for the Recana thrombectomy catheter system, which is...
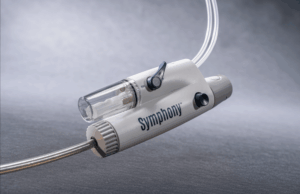
SYMPHONY-PE trial demonstrates positive efficacy, efficiency, and safety results
Imperative Care has announced efficacy and safety results from the pivotal SYMPHONY-PE trial evaluating the company’s Symphony thrombectomy system in the treatment of acute...
Imperative Care receives US FDA 510(k) clearance for Symphony thrombectomy system
Imperative Care today announced US Food and Drug Administration (FDA) 510(k) clearance of its Symphony thrombectomy system to treat pulmonary embolism (PE).
This clearance expands the...
Inari Medical, now part of Stryker, launches InThrill thrombectomy system
Inari Medical—now part of Stryker—has today announced the launch of its next-generation InThrill thrombectomy system.
The company has stated in a recent press release that...
First patient treated with InterVene’s Recana thrombectomy catheter system
InterVene announced today that the first patient has been treated with its Recana thrombectomy catheter system for venous in-stent restenosis (ISR).
The company shares...
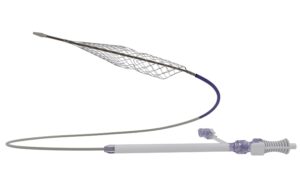
Endovascular Engineering’s ENGULF pivotal trial completes patient enrolment
Endovascular Engineering (E2) has completed patient enrolment in the pivotal cohort of its ENGULF trial, involving the Hēlo pulmonary embolism (PE) thrombectomy system.
The investigational...
Imperative Care announces completion of enrolment in the SYMPHONY-PE study
Imperative Care today announced the completion of patient enrolment in its SYMPHONY-PE study, a pivotal investigational device exemption (IDE) trial evaluating the safety and...
JETi registry reports effective thrombus reduction in lower extremity DVT
Recently presented at the Society of Interventional Radiology (SIR) annual scientific meeting (29 March–2 April, Nashville, USA), data from the Jet Enhanced Thrombectomy intervention...
Imperative Care expands Symphony thrombectomy portfolio with new US FDA approval
Imperative Care today announced US Food and Drug Administration (FDA) 510(k) clearance of the 82cm version of its Symphony 16Fr catheter, the company’s latest...
Endovascular Engineering raises US$42 million in Series B financing
Endovascular Engineering (E2) has secured US$42 million in an oversubscribed Series B financing to advance its next-generation clot removal technology platform for venous thromboembolism...
Akura Medical enrols first patient in QUADRA-PE study
Akura Medical has announced today the first patient enrolment in the QUADRA-PE study evaluating the Katana thrombectomy system in patients with acute pulmonary embolism...
Argon Medical enrols first patient in CLEAN-PE study
Argon Medical announces the first patient enrolment in the CLEAN-PE study. The prospective, multicentre CLEAN-PE study aims to evaluate the safety and efficacy of...
New analysis shows patients treated with Penumbra’s CAVT technology for PE...
Penumbra has announced new data that demonstrate patients with intermediate-risk pulmonary embolism (PE) treated with Penumbra's computer-assisted vacuum thrombectomy (CAVT) technology have a shorter...
Inquis Medical announces completion of US$40 million series B financing
Inquis Medical has announced the successful closure of its US$40 million series B financing round. This round was led by Marshall Wace, a globally...
TCT 2024: PEERLESS trial finds large-bore mechanical thrombectomy superior for intermediate-risk...
Findings from the first international randomised controlled trial (RCT) to compare patient outcomes following treatment with large-bore mechanical thrombectomy (LBMT) versus catheter-directed thrombolysis (CDT)...
Surmodics receives US FDA 510(k) clearance for Pounce XL thrombectomy system
Surmodics has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance for its Pounce XL thrombectomy system.
The Pounce XL thrombectomy...
Argon Medical announces launch of peripheral venous thrombectomy system
Argon Medical has today announced the launch of the Cleaner Vac thrombectomy system for the removal of blood clot from the peripheral venous vasculature.
The...
Penumbra receives CE mark for Lightning Flash 2.0 and Lightning Bolt...
Penumbra today announced it has secured CE mark in Europe for two computer-assisted vacuum thrombectomy (CAVT) technologies—Lightning Flash 2.0 and Lightning Bolt 7.
“Based on...
Thrombectomy gold rush set to follow ATTRACT study, experts argue
At the recent Endo Vascular Access (EVA) meeting (14–15 June, Patras, Greece), Ziv Haskal (University of Virginia School of Medicine, Charlottesville, USA) spoke to...
Aspirex achieves 78% two-year primary patency rate in P-MAX postmarket observational...
At the Leipzig Interventional Course (LINC) 2024 (May 28–31, Leipzig, Germany), Michael Lichtenberg (Arnsberg Vascular Clinic, Arnsberg, Germany) shared for the first time a...
Novel thrombectomy system demonstrates positive safety and feasibility results in treating...
Late-breaking data from the ENGULF trial showed that a novel dual-action thrombectomy device was effective and safe in treating acute pulmonary embolism (PE). The...
Expanse Ice aspiration system receives US FDA clearance for vessels of...
Expanse Ice recently announced that its Ice aspiration system has received 510(k) clearance from the US Food and Drug Administration (FDA).
A press release notes...
CX 2024: Dive into the Venous and Lymphatic Podium Firsts
Manj Gohel (Cambridge, UK), member of the Venous Executive Board, parachutes straight into the CX Venous and Lymphatics Controversies Programme to highlight the VenaSeal SPECTRUM...
Large administrative databases enter crosshairs as comparative analysis shows percutaneous mechanical...
The pitfalls of large administrative databases came to the fore during the presentation of a propensity-match scoring analysis demonstrating that percutaneous mechanical thrombectomy (MT)...
Innova Vascular announces successful early commercial use of Laguna thrombectomy system...
Innova Vascular has announced successful early commercial use of the company's Laguna thrombectomy system. Physicians at UCLA Medical Center in Los Angeles, USA, and...
Akura thrombectomy system for PE appears safe and demonstrates reduction of...
Results from a first-in-human, prospective, single-arm study of the Akura Medical thrombectomy system (Akura Medical) for pulmonary embolism (PE) were revealed this week at...
Mechanical thrombectomy: ClotTriever offers “extended window” for DVT treatment
This advertorial, sponsored by Inari Medical, is only available in selected countries and geographies.
During a recent webinar hosted by Inari Medical, a multidisciplinary group...
Inari Medical announces commercial launch of RevCore and Triever16 Curve for...
Inari Medical today announced the launch of two new purpose-built products, the RevCore thrombectomy catheter, and the Triever16 Curve catheter.
According to a company press...
RESCUE trial shows reduction in segmental and main pulmonary artery occlusions
Robert A Lookstein, who is executive vice chair, Diagnostic, Molecular and Interventional Radiology at the Icahn School of Medicine at Mount Sinai Hospital (New...
AVF 2023: One-year analysis of CLOUT registry reveals “significant and sustained”...
Interim one-year outcomes from the multicentre, prospective, single-arm CLOUT registry investigating use of the ClotTriever thrombectomy system (Inari Medical) in all-comer patients with deep...
Penumbra and Asahi Intecc partner to introduce Indigo system to Japan
Penumbra and Asahi Intecc, a Japanese medical device manufacturer, announced that they will collaborate to introduce Penumbra’s Indigo aspiration system into the Japanese market...
Penumbra announces the European launch of the Indigo system with Lightning...
Penumbra has announced that its Indigo aspiration system with Lightning 7 and Lightning 12 have secured CE mark and are now commercially available in...
Cordis makes strategic investment in E2, a developer of next-generation thrombectomy...
Cordis has announced a strategic investment venture that will expand the scope of the global cardiovascular technology company into the venous thromboembolism (VTE) market...
AVF 2022: Six-month CLOUT data indicate ClotTriever can effectively remove full...
Six-month outcomes from the ongoing CLOUT registry demonstrate the “safety and efficacy” of the ClotTriever thrombectomy system (Inari Medical) in a real-world deep vein...
Cardiovascular Systems partners with Innova Vascular to develop full line of...
Cardiovascular Systems Inc (CSI) recently announced it has partnered with Innova Vascular (Innova) to develop a full line of novel thrombectomy devices.
According to a...
Akura Medical closes US$25M Series A1 funding round
Akura Medical has announced the closing of its $25M Series A1 financing, which will be used to support the development of its next-generation thrombectomy device.
The financing...
Boston Scientific announces agreement to acquire Devoro Medical
Today, Boston Scientific announced an agreement to acquire Devoro Medical, developer of the Wolf thrombectomy platform. The non-console and lytic-free Wolf technology targets and...
Surmodics builds thrombectomy portfolio with acquisition of Vetex Medical
Surmodics recently announced that it has acquired privately-held Vetex Medical Limited. The Galway, Ireland-based medical device developer and manufacturer has focused exclusively on venous...
Vetex Medical announces positive one-year outcomes for ReVene thrombectomy catheter
Vetex Medical has announced positive one-year outcomes from a European clinical study of the ReVene thrombectomy catheter. In patients with iliofemoral vein thrombus, the...
Philips launches QuickClear mechanical thrombectomy system for blood clot removal in...
Royal Philips has announced the launch of the QuickClear mechanical thrombectomy system. The single-use system delivers an all-in-one aspiration pump and catheter to remove...
At six months, adjunctive mechanical thrombectomy associated with higher patency and...
A recent systematic review and meta-analysis showed that, in patients with iliofemoral deep vein thrombosis (DVT), percutaneous mechanical thrombectomy was associated with a higher...
FDA clears Aspire MAX mechanical thrombectomy system
Control Medical Technology has announced that the US Food and Drug Administration (FDA) has cleared its Aspire MAX 7 – 11F mechanical thrombectomy...
First look at CLOUT registry reveals over 75% of DVT patients...
David J. Dexter, (Sentara Healthcare, Norfolk, VA, USA), principal investigator of the CLOUT registry using the ClotTriever Mechanical Thrombectomy System (Inari Medical) for treatment...
Clinical data on the ClotTriever system demonstrates efficacy for DVT treatment
Inari Medical has announced the presentation of early outcomes from the ClotTriever Outcomes Registry (CLOUT) using the ClotTriever Mechanical Thrombectomy System for treatment of...
New developments in treatment of deep vein thrombosis
An analysis of ATTRACT has recently been published in the journal Circulation, in which the authors examine the effect of pharmacomechanical catheter-directed therapy (PCDT) in ATTRACT patients...
AngioJet thrombectomy can reduce dose and duration of lysis compared to...
Anna Louise Pouncey revealed at the European Venous Forum annual meeting (EVF; 28–30 June, Athens, Greece) the findings of the GSTT venous team (Guy’s...
NexGen Medical Systems launches XCOIL large vessel thrombectomy system
NexGen Medical Systems, Inc., a US medical device company, has announced the successful completion of the first human use of their XCOIL large vessel (18mm)...