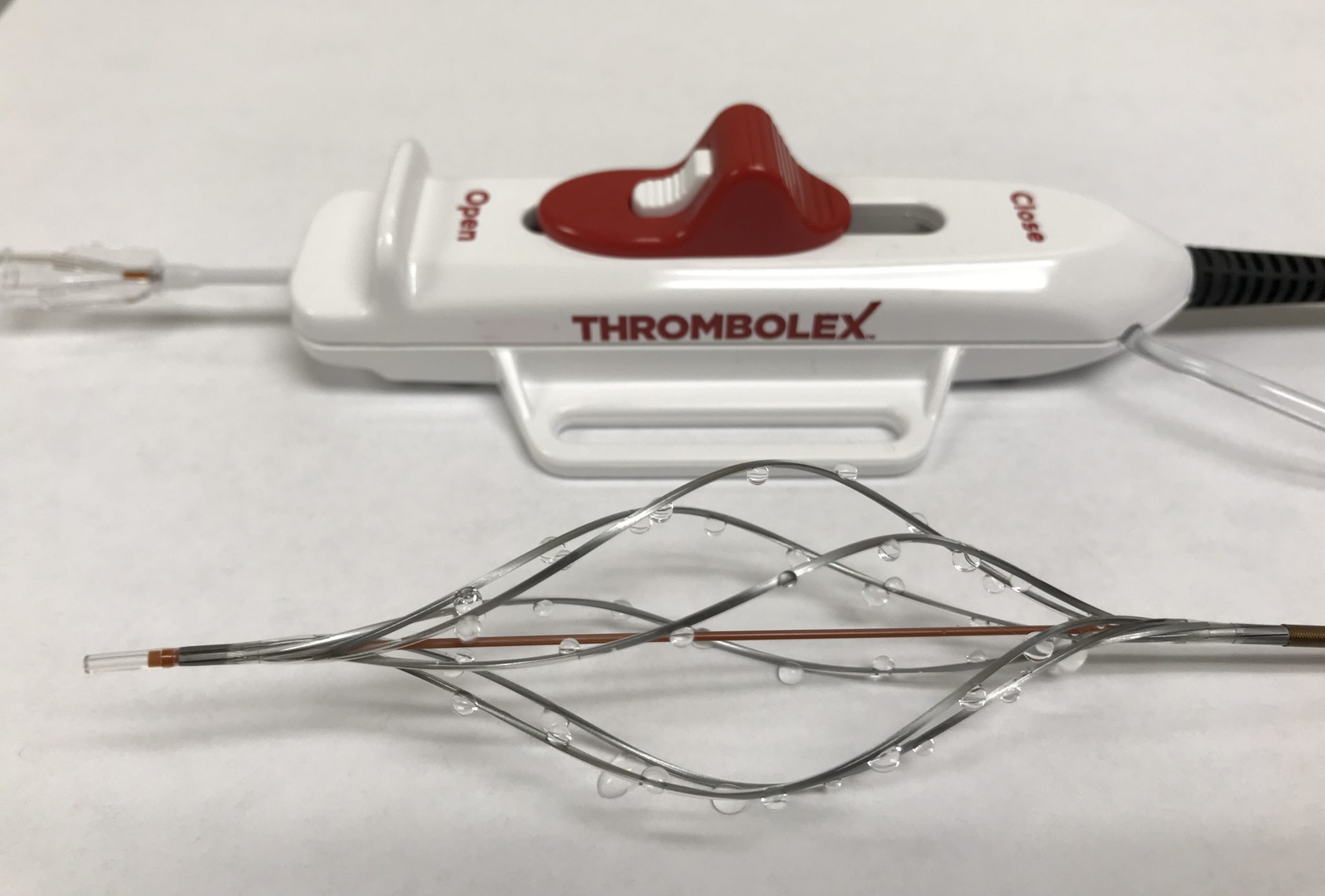
 Thrombolex has announced that the US Food and Drug Administration (FDA) has cleared the Bashir Endovascular Catheter (BEC) for the controlled and selective infusion of physician-specified fluids, including thrombolytics, into the peripheral vasculature, and the Bashir N-X Endovascular Catheter (BEC N-X) for the controlled and selective infusion of physician-specified fluids into the peripheral and pulmonary artery vasculature. The company has also received FDA clearance to begin an early feasibility study evaluating the Bashir platform for treatment of pulmonary embolism.
Thrombolex has announced that the US Food and Drug Administration (FDA) has cleared the Bashir Endovascular Catheter (BEC) for the controlled and selective infusion of physician-specified fluids, including thrombolytics, into the peripheral vasculature, and the Bashir N-X Endovascular Catheter (BEC N-X) for the controlled and selective infusion of physician-specified fluids into the peripheral and pulmonary artery vasculature. The company has also received FDA clearance to begin an early feasibility study evaluating the Bashir platform for treatment of pulmonary embolism.
The BEC is the only pharmacomechanical catheter cleared by the FDA that incorporates a physician controlled distal segment with six expandable mini-infusion catheters to deliver physician specified fluids in precise locations in the patient’s peripheral vasculature.
Riyaz Bashir, professor of Medicine, Director of Vascular and Endovascular Medicine at Temple University Hospital (Philadelphia, USA) said, “My inspiration for the BEC platform technology was to develop a device that I hoped would provide better treatment outcomes by rapid restoration of blood flow through the thrombus thereby enhancing both endogenous and exogenous fibrinolysis.” Bashir went on to say, “acute venous thromboembolic (VTE) disease has become a significant public health concern in the USA, with approximately 900,000 patients diagnosed and causing up to 100,000 deaths each year.”
Marvin Woodall, Chairman & CEO and co-founder of Thrombolex said, “Our dedicated team is proud to have received FDA clearance for our first two catheter-directed thrombolysis (CDT) products to treat patients suffering from acute VTE disorders”. Michael Cerminaro, president, COO and co-founder of Thrombolex commented, “Our first two products feature unique platform technologies which will be included in our significant pipe-line of new products currently being developed.”
Brian Firth, chief scientific officer of Thrombolex, said, “We recently received FDA clearance to begin an Early Feasibility Study (EFS) to demonstrate the safety and feasibility of the Bashir platform technology in the treatment of acute pulmonary embolism (PE). The protocol requires the use of the BECs expandable mini-infusion catheters to deliver low-doses of r-tPA directly into an acute PE.” Firth further stated, “The goal is to minimises the risk of major bleeding complications (including intracranial haemorrhage) associated with systemic lytic administration.”
Further clinical data will provide new insights into the advantages of rapid restoration of blood flow. Thrombolex believe that harnessing the endogenous lytics in the patient’s own blood in a synergistic manner with a lower dose of exogenous lytic has the potential to accelerate clot lysis and more effectively reduce the total thrombus burden. This should reduce the incidence of chronic pulmonary thromboembolic disease and post thrombotic syndrome.
The BEC and the BEC N-X are currently only available in the USA. Plans for distribution outside the USA are forthcoming. Thrombolex representatives will be present at the Society for Cardiovascular Angiography & Interventions (SCAI) Conference in Las Vegas on May 20-22, 2019 and invite physicians to visit their exhibit for additional information.