LimFlow has announced completion of enrolment of the original 10-patient cohort in the US feasibility study of the LimFlow percutaneous deep vein arterialisation system.
The company also announced that the US FDA has accepted the company’s proposal to expand the feasibility study by an additional 15 patients and three new sites, bringing the total number of patients enrolled in the feasibility study to 25 US subjects and six US institutions.
“This is the biggest advance I have seen for people at risk of amputation in my 25 years in practice,” said Daniel Clair, chair of the Department of Surgery at the University of South Carolina and the Palmetto Health-USC Medical Group, Columbia, USA, who performed the tenth case in the feasibility study on 25 January. “In the past, the only thing we had to offer these no-option patients was a segmental amputation of parts of their foot, and most patients ended up losing their entire foot. Major amputation has a very poor overall prognosis in terms of mortality and quality of life for patients and must be improved.”
The FDA also accepted the LimFlow System into its Breakthrough Device Program, a designation previously known as the Expedited Access Pathway (EAP). The designation is intended to speed patient access to breakthrough technologies that provide for more effective treatment of life-threatening or irreversibly debilitating diseases, for which no approved or cleared treatment exists or that offers significant advantages over existing approved or cleared alternatives.
“We are excited to be among the first technologies treating lower extremity disease to be granted access to the FDA’s Breakthrough Device Program,” said LimFlow CEO Dan Rose. “Their decision recognises the novelty of our system and the promise it holds in treating the epidemic of critical limb ischemia. Over 100,000 patients in the USA have a major ischaemic lower limb amputation every year and we look forward to closely collaborating with the FDA to soon bring the LimFlow technology to US patients with no good options today.”
To relieve the symptoms of critical limb ischaemia today, patients are often treated with angioplasty or open bypass surgery. In many late-stage patients, however, neither option is feasible due to extensive disease in the target arteries or other anatomical constraints, the press release explains.
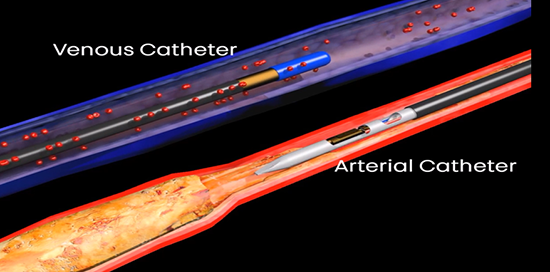
The LimFlow System uses proprietary ultrasound-guided catheters and covered nitinol stents designed to restore perfusion to the ischaemic foot by bypassing diseased arteries and diverting blood flow into the tibial vein to vascularise the foot. Achieving tissue perfusion may relieve rest pain, promote chronic wound healing, reduce major amputations and restore mobility for patients, the release concludes.












