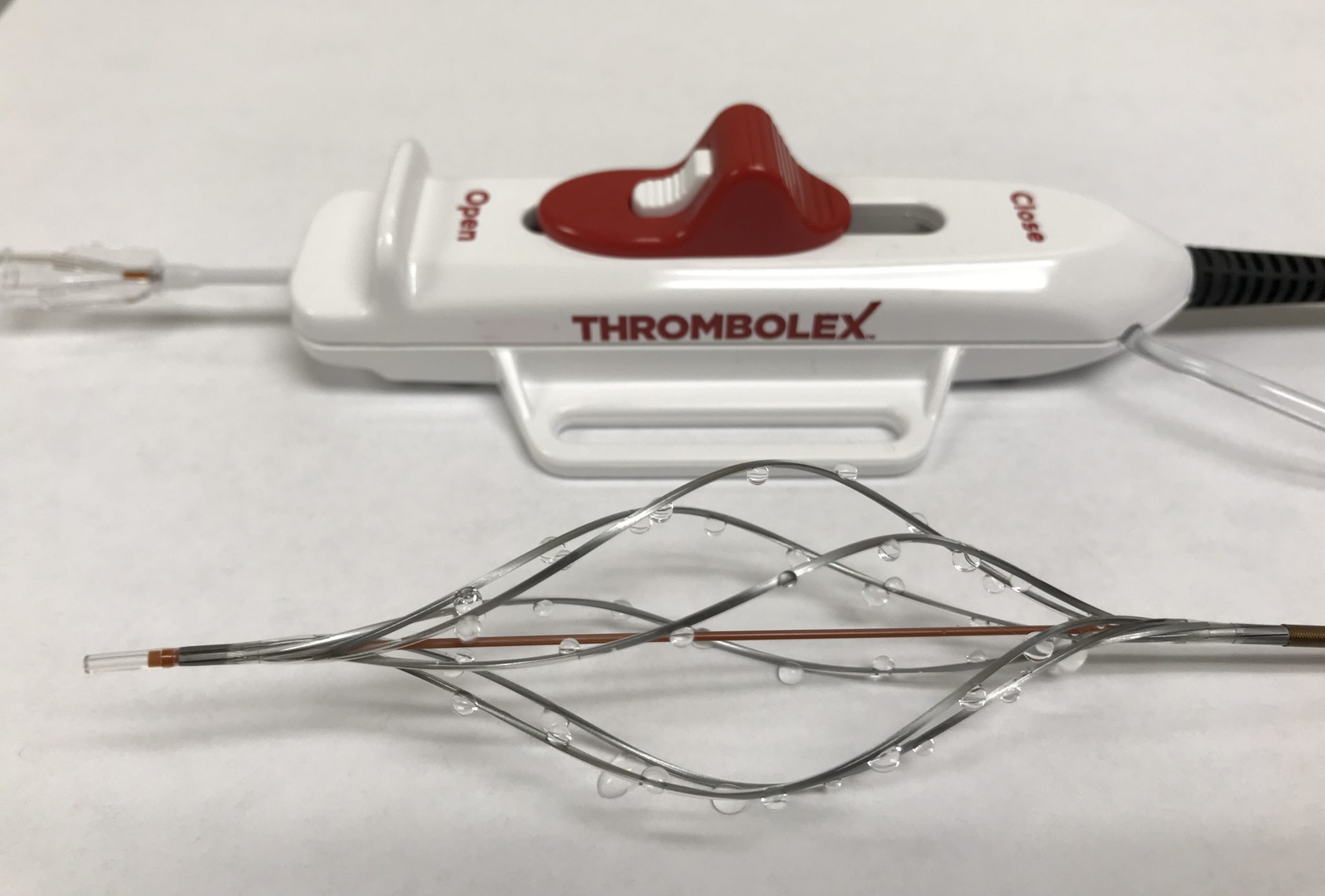
 Thrombolex has announced the enrolment of the first patient in their Early Feasibility and Safety Study, investigating the Bashir Endovascular Catheter for the treatment of submassive pulmonary embolism (PE). The device was designed to quickly and safely dissolve thrombus and restore blood flow in patients with thrombotic vascular occlusions.
Thrombolex has announced the enrolment of the first patient in their Early Feasibility and Safety Study, investigating the Bashir Endovascular Catheter for the treatment of submassive pulmonary embolism (PE). The device was designed to quickly and safely dissolve thrombus and restore blood flow in patients with thrombotic vascular occlusions.
The Bashir catheter includes an expandable infusion basket comprised of six mini-infusion catheters with 48 precision, laser-drilled infusion holes. When expanded, it can promptly restore blood flow to enable endogenous lytics in the blood to help accelerate clot lysis. In this investigational study the device is used to administer thrombolytics through multiple cross-sections of the culprit clot to enhance clot dissolution.
“We are pleased to have been the first site to enrol a patient in the Thrombolex Early Feasibility and Safety Study,” said Parth Rali, assistant professor, Thoracic Medicine and Surgery at Lewis Katz School of Medicine at Temple University in Philadelphia, USA. “The catheter removed a large volume of clot from the pulmonary arteries efficiently, and safely restored blood flow, that resulted in a very rapid improvement in the patient’s overall clinical status.”
In this patient with a critical, large submassive PE that severely compromised blood flow to both lungs, a total of 14mg of r-tPA was administered over eight hours. “There was a dramatic improvement in the patient’s haemodynamic state and oxygenation as well as evidence of a marked reduction in clot burden as compared to what has been seen in other catheter-directed thrombolysis studies using a similar dose of r-tPA,” said Riyaz Bashir, director of Vascular and Endovascular Medicine at Temple University Hospital. “We believe that the Bashir Endovascular Catheter may represent a quantum leap in the treatment of venous thromboembolism patients, not just acutely, but also in terms of their longer-term health status,” said Brian Firth, chief scientific officer at Thrombolex.
“Thrombolex is focused on developing more advanced therapies for treatment of patients suffering with venous thromboembolic (VTE) disorders,” said Mike Cerminaro, president & COO of Thrombolex. “We are passionate about our focus because PE is a common disease, which is the third highest cause of cardiovascular mortality and is responsible for up to 180,000 deaths annually in the USA alone.”
“Currently available infusion catheters used to treat PE and other large clots are not able to rapidly restore blood flow through the thrombus and allow for only limited radial diffusion of exogenously administered thrombolytics like r-tPA into a clot,” said Marv Woodall, chairman & CEO of Thrombolex. The Thrombolex investigational trial is designed to examine if lower doses of thrombolytic via the Bashir Endovascular Catheter can be administered much more effectively over less time and thereby minimise major bleeding complications. This investigational study is a 10-patient prospective, multicentre clinical study to evaluate feasibility and safety of the catheter to treat patients with intermediate-risk PE. It represents the first in a series of planned clinical trials to evaluate this new platform technology that has been developed by Thrombolex.












