Tag: pulmonary embolism
PERTs improve outcomes but “magic formula” does not exist, LINC audience...
In a session at the 2026 Leipzig Interventional Course (LINC; 27–31 January, Leipzig, Germany) dedicated to pulmonary embolism (PE) management, Sahil Parikh (Columbia University...
Investigator-led initiative supporting PE research launched
AngioDynamics and The PERT Consortium have announced the launch of the ALPHA-PE Research Fund, an investigator-led initiative supporting independent pulmonary embolism (PE) research.
A press...
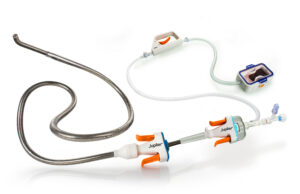
Enrolment in SPIRARE II pivotal trial of embolectomy system for pulmonary...
Jupiter Endovascular today announced completion of patient enrolment in the SPIRARE II pivotal trial evaluating the Vertex pulmonary embolectomy system in patients with acute,...
SonoVascular completes first close of Series A preferred stock financing
SonoVascular, developer of the SonoThrombectomy system for the treatment of venous thromboembolism (VTE), has completed the first close of its US$6 million Series A...
Penumbra unveils latest iteration of thrombectomy system for venous thromboembolism
Penumbra has launched the Lightning Flash 3.0 computer-assisted vacuum thrombectomy (CAVT) system, a development that includes "significant upgrades" to the venous thromboembolism (VTE) platform,...
PE thrombectomy: ENGULF pivotal study meets safety and efficacy endpoints
Full results from the ENGULF pivotal study of the Hēlo thrombectomy system (Endovascular Engineering) in acute intermediate risk pulmonary embolism (PE) patients demonstrated a...
CAVT with anticoagulation significantly improves functional outcomes in PE patients, RCT...
Mechanical thrombectomy by means of computer-assisted vacuum thrombectomy (CAVT) using the 16Fr Lightning Flash system (Penumbra) along with anticoagulation for acute-to-intermediate pulmonary embolism (PE)...
Interim RAPID-PE study reveals ‘excellent’ safety data in first 50 patients
Prespecified interim analysis data from the first 50 patients in RAPID-PE have demonstrated "excellent safety" and "remarkably efficient lab times" in patients receiving on-the-table...
Study finds PERT-advised mechanical thrombectomy reduces mortality in PE
Results from a retrospective observational cohort study demonstrate reduced 30-day all-cause mortality, length of hospital stay and supplemental oxygen use at discharge in patients...
STORM-PE finds mechanical thrombectomy superior to anticoagulation alone
The use of mechanical thrombectomy, specifically computer-assisted vacuum thrombectomy (CAVT) using the 16Fr Lightning Flash system (Penumbra), with anticoagulation achieves superior reduction in right...
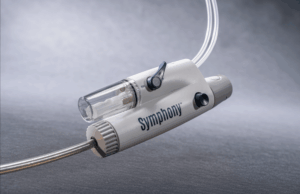
Imperative Care receives US FDA 510(k) clearance for Symphony thrombectomy system
Imperative Care today announced US Food and Drug Administration (FDA) 510(k) clearance of its Symphony thrombectomy system to treat pulmonary embolism (PE).
This clearance expands the...
First patient enrolled in RECOVER-AV trial evaluating AngioDynamics’ AlphaVac F18⁸⁵ system...
AngioDynamics recently announced the first patient enrolment in RECOVER-AV—a prospective, multicentre, multinational, single-arm study evaluating the AlphaVac multipurpose mechanical aspiration (MMA) F1885 system in...
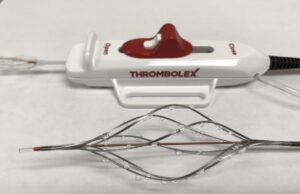
RESCUE II results on Thrombolex’s Bashir endovascular catheter for PE published
Thrombolex recently announced the publication of RESCUE-II study results in JACC: Advances.
The RESCUE-II study was a single-centre, prospective study evaluating the safety and...
Aventus thrombectomy system gains FDA clearance for PE treatment
Inquis Medical has announced that its Aventus thrombectomy system has received 510(k) clearance from the US Food and Drug Administration (FDA) for an expanded...
EVC 2025: PE interventions “should be part of the armamentarium of...
Makis Avgerinos (Athens, Greece), co-director of the venous programme for the European Vascular Course (EVC), speaks to Venous News at this year’s meeting (9–11...
Inquis Medical’s Aventus thrombectomy system found safe and effective for patients...
Inquis Medical recently announced results from its AVENTUS trial evaluating the safety and efficacy of the company’s Aventus thrombectomy system.
The results were presented by...
New analysis details learning curve effect behind ‘significant improvement’ in performance...
A multisite hospital system analysis of the safety and procedural learning curve behind the performance of percutaneous mechanical thrombectomy for pulmonary embolism (PE) shows...
Endovascular Engineering raises US$42 million in Series B financing
Endovascular Engineering (E2) has secured US$42 million in an oversubscribed Series B financing to advance its next-generation clot removal technology platform for venous thromboembolism...
Akura Medical enrols first patient in QUADRA-PE study
Akura Medical has announced today the first patient enrolment in the QUADRA-PE study evaluating the Katana thrombectomy system in patients with acute pulmonary embolism...
Argon Medical enrols first patient in CLEAN-PE study
Argon Medical announces the first patient enrolment in the CLEAN-PE study. The prospective, multicentre CLEAN-PE study aims to evaluate the safety and efficacy of...
Flow Medical secures US$5 million seed funding to advance pulmonary embolism...
Flow Medical today announced the closing of an oversubscribed US$5 million seed funding round, which includes US$2 million previously secured from friends and family....
New analysis shows patients treated with Penumbra’s CAVT technology for PE...
Penumbra has announced new data that demonstrate patients with intermediate-risk pulmonary embolism (PE) treated with Penumbra's computer-assisted vacuum thrombectomy (CAVT) technology have a shorter...
Inquis Medical announces completion of US$40 million series B financing
Inquis Medical has announced the successful closure of its US$40 million series B financing round. This round was led by Marshall Wace, a globally...
TCT 2024: PEERLESS trial finds large-bore mechanical thrombectomy superior for intermediate-risk...
Findings from the first international randomised controlled trial (RCT) to compare patient outcomes following treatment with large-bore mechanical thrombectomy (LBMT) versus catheter-directed thrombolysis (CDT)...
Thrombolex begins enrolment in RAPID-PE study of Bashir catheter
Thrombolex has announced the enrolment of the first two patients in the RAPID-PE study using the Bashir endovascular catheter for the treatment of acute...
Kush Desai
“One of the great and simultaneously worrisome things about venous disease is that we’re still in the first generation of our understanding of pathophysiology...
Mechanical thrombectomy shifts the needle toward interventional management of PE and...
This advertorial is sponsored by Inari Medical.
Evaluating the current standard of care for the management of acute pulmonary embolism (PE) and deep vein thrombosis...
Ensuring the right treatment for patients with PE and DVT
In this short video, Michael Kostrzewa (Baden, Switzerland) and Rashid Akhtar (London, UK) discuss the latest approaches to the treatment of acute pulmonary embolism...
Inari Medical updates ClotTriever XL IFU amid US FDA recall notice
The US Food and Drug Administration (FDA) has issued a Class I recall—the most serious type—for Inari Medical's ClotTriever XL, 30mm device due to...
Endovascular Engineering appoints Dan Rose as chief executive officer
Endovascular Engineering (E2), which specialises in the advancement of clot removal technologies for venous thromboembolism (VTE), has named Dan Rose as chief executive officer...
Thrombolex and Aidoc announce strategic partnership to advance breakthrough PE treatment
Thrombolex and Aidoc have announced a strategic partnership aimed at revolutionising the treatment of acute pulmonary embolism (PE), a press release reports.
This strategic collaboration,...
Thrombectomy gold rush set to follow ATTRACT study, experts argue
At the recent Endo Vascular Access (EVA) meeting (14–15 June, Patras, Greece), Ziv Haskal (University of Virginia School of Medicine, Charlottesville, USA) spoke to...
INSIGHTS-SVT study highlights importance of individual treatment strategies
A recent study published in the Journal of Vascular Surgery: Venous and Lymphatic Disorders looked at varicose vein surgery after acute isolated superficial vein...
EU project develops portable diagnostic device for the early detection of...
Deep vein thrombosis (DVT) is a significant health risk. In about half of patients, the blood clot breaks away from the vein wall and...
Gloria Salazar
“There are a lot of patients walking out there who are not being treated. So you can only imagine the impact that we can...
Women and black patients less likely to receive catheter-based treatment for...
New data from the REAL-PE analysis investigated catheter-based pulmonary embolism (PE) treatment, showing women and Black people were less frequently treated with minimally invasive...
Penumbra announces FDA clearance of Lightning Flash 2 for the treatment...
Penumbra has announced the US Food and Drug Administration (FDA) clearance and launch of Lightning Flash 2, the next generation computer assisted vacuum thrombectomy (CAVT)...
FlowTriever device found to dramatically improve breathing among intermediate-risk acute pulmonary...
SCVS 2024 saw Zachary AbuRahma, DO, an assistant professor of vascular surgery at West Virginia University and the Charleston Area Medical Center in Charleston,...
AngioDynamics receives 510(k) clearance for AlphaVac F1885 System in treatment of...
AngioDynamics has announced that the United States Food and Drug Administration (FDA) has cleared their AlphaVac F1885 system for the treatment of pulmonary embolism...
FDA clears AI-driven detection tool for pulmonary embolism
Medical imaging AI company Avicenna.AI has announced 510(k) clearance from the US Food and Drug Administration (FDA) for its CINA-iPE artificial intelligence (AI)-powered tool that...
Adoption of AI into workflow associated with faster time to assessment...
An abstract presented at the 2024 American Venous Forum (AVF) annual meeting (March 3–6) revealed that, in the treatment of patients with pulmonary embolism...
Large administrative databases enter crosshairs as comparative analysis shows percutaneous mechanical...
The pitfalls of large administrative databases came to the fore during the presentation of a propensity-match scoring analysis demonstrating that percutaneous mechanical thrombectomy (MT)...
Enrollment complete in APEX-AV study of mechanical aspiration system for acute...
Patient enrollment is now complete in the APEX-AV trial evaluating the safety and efficacy of the AlphaVac F1885 (AngioDynamics) multipurpose mechanical aspiration system for...
Computer-assisted vacuum thrombectomy system for patients with pulmonary embolism demonstrates ‘rapid,...
Patients undergoing computer-assisted vacuum thrombectomy with the Indigo Aspiration System (Penumbra) for pulmonary embolism (PE) showed 2.7% rates of both major adverse events (MAEs)...
CX 2024 programme seeks to challenge venous controversy ‘head on’ to...
Forecasting their Charing Cross (CX) 2024 venous programme highlights, executive board members Manj Gohel (London, United Kingdom), Erin Murphy (Charlotte, USA) and Stephen Black...
Akura thrombectomy system for PE appears safe and demonstrates reduction of...
Results from a first-in-human, prospective, single-arm study of the Akura Medical thrombectomy system (Akura Medical) for pulmonary embolism (PE) were revealed this week at...
REAL-PE demonstrates statistically significant lower major bleeding rates with Ekos system...
Data from the REAL-PE study were presented this week at TCT 2023 (23–26 October, San Francisco, USA) demonstrating that patients treated for pulmonary embolism...
Thrombolex announces new insights from the RESCUE trial with the Bashir...
Thrombolex has announced never-before-reported major reductions in obstruction in all of the segmental pulmonary arteries (PA), based on independent core lab data analysis of...
Aidoc’s AI solution for pulmonary embolism clinically proven to reduce hospital...
Research in the advancement of artificial intelligence (AI)-driven pulmonary embolism (PE) care was unveiled recently during the 9th Annual Pulmonary Embolism Symposium (Sept. 21–23)...
New clinical data support Viz.ai solution for improved pulmonary embolism detection...
Viz.ai has announced new clinical data supporting advancements in pulmonary embolism (PE) detection. Two studies have demonstrated the real-world clinical efficacy of Viz.ai's PE...
The pulmonary arterial tree—it is time we branch out
Nicolas J Mouawad (McLaren Health System, Bay City, USA) urges vascular surgeons to “get out of their comfort zone” and become more involved in...
Inari Medical announces commercial launch of RevCore and Triever16 Curve for...
Inari Medical today announced the launch of two new purpose-built products, the RevCore thrombectomy catheter, and the Triever16 Curve catheter.
According to a company press...
FLAME, FLASH and ongoing PEERLESS trials collect “compelling” data for FlowTriever...
Speaking on the FLAME, FLASH and PEERLESS trials that each collected data on the FlowTriever (Inari Medical) device for the treatment of pulmonary embolism...
Akura Medical announces successful first-in-human use of its mechanical thrombectomy platform
Akura Medical announced today it has initiated its first-in-human clinical study of the Akura mechanical thrombectomy platform. A press release notes that the Akura...
AVF 2023: FLASH registry suggests “favourable safety and effectiveness profile” in...
Among 61 high-risk pulmonary embolism (PE) patients followed through to the 30-day visit in the US cohort of the FLASH registry, no mortalities were...
Viz.ai to expedite patient enrolment in NIH-funded PE-TRACT clinical trial
Viz.ai has announced it will use its Viz Recruit platform to optimise patient enrolment for the National Institutes of Health (NIH)-funded Pulmonary embolism—thrombus removal...
New study demonstrates IVC filters “safe and effective” in treating venous...
Few adverse events are connected to the use of inferior vena cava (IVC) filters to help prevent deep vein blood clots from developing into...
Inari Medical announces first patient enrolment in DEFIANCE trial of the...
Inari Medical has announced that the first patient has been enrolled in DEFIANCE, a prospective randomised controlled trial (RCT) comparing the clinical outcomes of...
FLASH results demonstrate “excellent safety profile” of the FlowTriever system in...
Results of the FLASH registry demonstrate the “excellent safety profile” of the FlowTriever system (Inari Medical) in 800 “real-world” patients. This is according to...
Penumbra and Asahi Intecc partner to introduce Indigo system to Japan
Penumbra and Asahi Intecc, a Japanese medical device manufacturer, announced that they will collaborate to introduce Penumbra’s Indigo aspiration system into the Japanese market...
RESCUE trial of the Bashir endovascular catheter demonstrates reduction in pulmonary...
Thrombolex today presented the final results of its National Institutes of Health (NIH)-sponsored RESCUE trial at TCT 2022 (16–19 September, Boston, USA).
This investigational...
Viz.ai receives FDA 510(k) clearance for automated RV/LV analysis algorithm
Viz.ai recently announced it has received US Food and Drug Administration (FDA) 510(k) clearance for an automated right ventricle (RV)/left ventricle (LV) ratio algorithm,...
New data underscore benefits of Penumbra’s Indigo for submassive pulmonary embolisms...
In a new subgroup analysis of the EXTRACT-PE trial, the Indigo aspiration system (Penumbra) was effective at improving clinical outcomes for submassive pulmonary embolism...
RapidAI receives FDA 510(k) clearance for PE Triage & Notification
RapidAI announced today that it has received US Food and Drug Administration (FDA) 510(k) clearance for its Rapid PE Triage & Notification product for...
Magneto Thrombectomy Solutions announces successful first-in-human results for treatment of pulmonary...
Magneto Thrombectomy Solutions (Magneto), a medical devices company developing thrombectomy solutions for the treatment of ischaemic stroke and pulmonary embolism, presented successful first-in-human (FIH)...
Penumbra announces the European launch of the Indigo system with Lightning...
Penumbra has announced that its Indigo aspiration system with Lightning 7 and Lightning 12 have secured CE mark and are now commercially available in...
Study finds increased risk of serious blood clots up to six...
A study from Sweden published by The BMJ recently finds an increased risk of deep vein thrombosis (DVT) up to three months after COVID-19 infection,...
Cordis makes strategic investment in E2, a developer of next-generation thrombectomy...
Cordis has announced a strategic investment venture that will expand the scope of the global cardiovascular technology company into the venous thromboembolism (VTE) market...
Anticoagulants effective for pulmonary embolism resolution in hospitalised COVID-19 patients
Anticoagulants alone are associated with a high rate of resolution of pulmonary embolism (PE) in patients with acute PE and COVID-19 infection. This was...
First patient enrolled in PEERLESS study of FlowTriever system
Inari Medical has announced that the first patient has been enrolled in PEERLESS—a prospective, randomised controlled trial (RCT) comparing the outcomes of patients with...
Thrombolex announces new interim data from RESCUE trial of the Bashir...
Thrombolex has announced positive results from the prespecified interim analysis of the first 62 evaluable pulmonary embolism (PE) patients enrolled in the investigational RESCUE...
Viz.ai launches two new AI-powered modules for pulmonary embolism and aortic...
Viz.ai has announced the US commercial launch of its AI-powered modules for pulmonary embolism and aortic disease. Debuting at VEITHsymposium 2021 (16–20 November, Orlando, USA), the...
Inari Medical announces six-month FLASH registry interim data
Inari Medical has announced positive acute and long-term interim results from the first 500 pulmonary embolism (PE) patients enrolled in the FlowTriever outcomes registry...
Positive data for the EkoSonic endovascular system presented at VIVA 2021
Boston Scientific announced positive results for the EkoSonic endovascular system (EKOS system) during a late-breaking clinical trial presentation at Vascular Interventional Advances (VIVA) 2021...
Thrombolex announces interim RESCUE results at VIVA 2021
Thrombolex recently announced the results of the RESCUE trial’s prespecified interim analysis, of the first 62 evaluable patients, in a late-breaking clinical trials session...
Inari Medical appoints pulmonologist Victor F Tapson as VP of Medical...
Inari Medical has announced that the company has appointed Victor F Tapson as vice president of Medical Affairs.
Tapson has devoted his medical career to...
Thromboprophylaxis with anticoagulants “likely effective” at preventing VTE post-vascular surgery, larger...
“Among patients undergoing vascular surgery, thromboprophylaxis with anticoagulants showed a trend towards reduced incidence of VTE when compared to placebo,” Tarek Haykal (Duke...
Boston Scientific initiates randomised controlled trial for the EkoSonic endovascular system
Boston Scientific has commenced enrolment in the HI-PEITHO clinical trial, a collaborative research study with the Pulmonary Embolism Response Team (PERT) Consortium and the...
Viz.ai and Avicenna.AI partner to launch AI-driven intelligent care coordination for...
Viz.ai has partnered with Avicenna.AI to enable intelligent care coordination and improve patient triage of patients suffering from pulmonary embolism (PE) and aortic disease.
Avicenna.AI’s US...
Thrombolex receives enhanced indication for use from the FDA
The US Food and Drug Administration (FDA) has enhanced the indications for use of the Bashir endovascular catheter (Thrombolex) and the Bashir Plus endovascular...
Propofol use during catheter-directed interventions for intermediate-risk PE associated with major...
A study suggests avoiding propofol for intraprocedural sedation during catheter-directed interventions (CDIs) for intermediate-risk pulmonary embolism (PE) because it can have detrimental effects. Propofol...
AI triage solution for PE and aortic dissection receives FDA clearance...
Medical imaging artificial intelligence (AI) specialist Avicenna.AI recently announced that it has received certification in the USA and European Union (EU) for CINA CHEST,...
Meta-analysis: IVC filters should be considered for certain patients at high...
In a recent meta-analysis, Yang Liu, Huan Lu (Henan Cancer Hospital, Zhengzhou, China), and colleagues found insufficient evidence to prove that inferior vena cava...
First-in-human results for the Bashir endovascular catheter published
Results of a first-in-human study to assess the safety and feasibility of the Bashir endovascular catheter (Thrombolex) for the treatment of acute intermediate-risk pulmonary...
Aidoc and Imbio partner to provide an AI solution to prioritise...
Aidoc and Imbio have announced a partnership intended to provide an end-to-end artificial intelligence (AI) solution for pulmonary embolism (PE), with the goal of ultimately...
Penumbra’s newest generation of Indigo aspiration system receives FDA clearance for...
Penumbra today announced US Food and Drug Administration (FDA) 510(k) clearance for expanded indication of the latest iteration of the Indigo aspiration system, Lightning...
Inari Medical announces presentation of 30-day follow-up results from first patients...
Inari Medical has announced follow-up results of the first 230 patients enrolled in its FLASH study. FLASH is a real-world registry to study the...
FLASH registry: Interim results “reinforce the excellent FlowTriever safety profile”
Catalin Toma (University of Pittsburgh, Pittsburgh, USA) recently presented interim results from the FLASH registry during a late-breaking session at TCT Connect (14–18 October,...
Systematic review finds high risk of deep vein thrombosis and pulmonary...
In a systematic review of the worldwide published data on venous thromboembolism (VTE) in COVID-19 patients, Cihan Ay, Stephan Nopp, and Florian Moik (Medical...
HOME-PE trial clarifies which patients with acute PE can be managed...
Patients with acute pulmonary embolism (PE) can be selected for home management using the sPESI score or the Hestia criteria, according to results of...
Study finds death rates from PE have been rising over the...
The American Heart Association (AHA) has announced that after nearly a decade of steady decline, the death rate for people with pulmonary embolism (PE)...
First patient enrolled in RESCUE trial of Thrombolex’s Bashir catheter
Thrombolex has announced that it has enrolled the first patient in its pivotal RESCUE trial for the treatment of patients with acute submassive pulmonary...
Compression stockings might not be needed to prevent blood clots after...
Compression stockings might be unnecessary to prevent blood clots in most patients undergoing non-emergency (elective) surgery, finds a clinical trial published by The BMJ...
Elder statesman of venous disease delivers golfing metaphor for fortitude, wellness
As a podium talk at the annual meeting of the American Venous Forum (VENOUS 2020; 3–6 March, Amelia Island, USA), it represented a bit...
First-in-human results for Bashir Endovascular Catheter meet safety and feasibility endpoints
Thrombolex has announced that results of its First-In-Human (FIH) trial confirm the early safety and feasibility of using the Bashir Endovascular Catheter for pharmacomechanical...
No difference in PE incidence between patients treated with IVC filters...
In a case-control study of patients with perceived contraindications to anticoagulation, who either received or did not receive inferior vena cava (IVC) filters, no...
Improved follow-up measures needed to fill knowledge gap in long-term PE...
“We need to check for signs of recurrence and functional impairment, and follow these patients much more closely,” urged Kenneth Rosenfield (Massachusetts General Hospital,...
SUNSET sPE thrombolysis trial completes enrolment phase
The “Standard versus ultrasound-assisted catheter thrombolysis for submassive pulmonary embolism” (SUNSET sPE) trial, a randomised, single-blinded clinical trial comparing ultrasound-assisted thrombolysis (USAT) to standard...
Penumbra wins FDA clearance for Indigo Aspiration System
Penumbra has announced US Food and Drug Administration (FDA) 510(k) clearance for expanded indication of the Indigo Aspiration System. As part of the system,...
First pulmonary embolism patient treated with “cyclical” aspiration system
Insera Therapeutics’ CLEAR aspiration system has been used for the first time to treat a (human) patient with acute pulmonary embolism (PE). CLEAR, “cyclical...
FDA approves first generics of Eliquis for prophylaxis of DVT and...
The US Food and Drug Administration (FDA) has announced the approval of two applications for the first generics of Eliquis (apixaban) tablets, to reduce...
Insera announces first-in-human treatment of patient with PE using CLEAR aspiration...
Insera Therapeutics announced recently that the first patient with acute pulmonary embolism has been treated with its flagship cyclical aspiration system, CLEAR. Earlier last...
New trial planned to rigorously test catheter-directed thrombolysis for submassive PE...
Clinically relevant outcomes are needed to measure catheter-directed therapy (CDT) against anticoagulation alone to treat submassive pulmonary embolism (PE) patients. Further, CDT’s ability to...
EXTRACT-PE trial of aspiration thrombectomy for acute pulmonary embolism meets efficacy...
It has been found by the EXTRACT-PE trial, a multicentre investigation conducted under an investigational device exemption (IDE) from the US Food and Drug...
Thrombolex announces first enrolment in early feasibility and safety study using...
Thrombolex has announced the enrolment of the first patient in their Early Feasibility and Safety Study, investigating the Bashir Endovascular Catheter for the treatment...
Catheter-directed thrombolysis treatment for PE may significantly reduce long-term costs with...
Catheter-directed thrombolysis improves outcomes and long-term cost efficiency for treatment of massive and submassive pulmonary embolism (PE), in comparison to systemic heparin administration. This...
SUNSET sPE thrombolysis trial presents interim analysis of ultrasound-guided versus standard...
The Standard vs ultrasound-assisted catheter thrombolysis for submassive pulmonary embolism (SUNSET sPE) trial, is an ongoing randomised, head-to-head, single-blinded clinical trial comparing ultrasound-assisted thrombolysis...
Inari Medical announces publication of FLARE IDE study results
Inari Medical has announced the publication of its 106-patient prospective multicentre FlowTriever Mechanical Pulmonary Embolectomy (FLARE) study for the treatment of intermediate-risk pulmonary embolism...
BTG acquires Novate Medical
BTG has announced it has acquired Novate Medical, a medical device company focused on the prevention of pulmonary embolism in patients at high risk...
Study proves need for anticoagulant strategy post discharge for hospitalised, at-risk...
Alex C Spyropoulos, from Feinstein Institute for Medical Research (New York, USA), has presented an anticoagulant treatment strategy to reduce non-fatal blood clots and...
PERT Consortium and BTG form strategic partnership
BTG has announced a strategic partnership with the PERT Consortium to advance the science of pulmonary embolism treatment and promote the implementation of PERT...
Direct anticoagulant for pulmonary embolism associated with reduced hospital time and...
New data from the MERCURY PE study have shown that patients with low-risk pulmonary embolism who were treated with the direct oral anticoagulant rivaroxaban,...
Cancer-associated VTE trial sees “lower rate of recurrent VTE with edoxaban...
Daiichi Sanyoko has announced the results from their Hokusai-VTE CANCER trial evaluating the direct oral anticoagulant edoxaban for treatment of venous thromboembolism (VTE) in...
Study to measure shorter duration EKOS therapy for pulmonary embolism is...
Global specialist healthcare company BTG has highlighted the commencement of the KNOCOUT PE study. The KNOCOUT PE study will measure how hospitals and patients...
Inari Medical announces completion of enrolment in the FLARE study for...
Inari Medical has announced that it has completed enrolment of its investigational device exemption (IDE) study. The FlowTriever Pulmonary Embolectomy Clinical Study (FLARE) study...
One-year SENTRY trial data offer a “a new perspective” on IVC...
Twelve-month results from the SENTRY trial evaluating the Sentry bioconvertible inferior vena cava (IVC) filter (Novate) have shown a 0% rate of symptomatic pulmonary...
Angel Catheter demonstrates reduction in clinically-significant and fatal pulmonary embolism
Bio2 Medical has announced the results of the Angel Catheter pivotal study published in the Journal of Vascular and Interventional Radiology. The Angel Catheter...
ACCESS PTS study demonstrates efficacy of EKOS therapy for post-thrombotic syndrome
The results of the ACCESS PTS trial have been presented at the Society for Vascular Medicine 28th Annual Scientific Sessions (14–17 June, New Orleans,...
OPTALYSE PE study demonstrates safety and efficacy of shorter, lower dose...
Results from the OPTALYSE PE trial have been presented at the American Thoracic Society International Conference in Washington, DC, USA. The results show that...
High-risk pulmonary embolism patients often go without most effective treatments
In a new study presented at the American College of Cardiology 66th Annual Scientific Session, researchers from the Perelman School of Medicine at the University of...