Highlights: -IN.PACT SFA two-year results "have potential to drive paradigm shift" in femoropopliteal lesion treatment -PERICLES study helps bring chimney technique "out of the shadows" -Endologix and TriVascular announce merger -Jeffrey P Carpenter: Protection devices -Peter H Lin: Thrombolysis...
By Athanasios D Giannoukas
Today, venous stenting plays an important role in the treatment of deep venous pathologies. The current indications for its use include acute iliofemoral thrombosis after catheter-directed or pharmacomechanical thrombolysis to resolve residual iliac stenosis, May-Thurner syndrome and...
According to new a study published in PLOS Medicine, a new clinical model can help predict the risk of venous thromboembolism (VTE) among patients with a leg case, enabling doctors to identify high risk cases.
Using data from three large cohorts,...
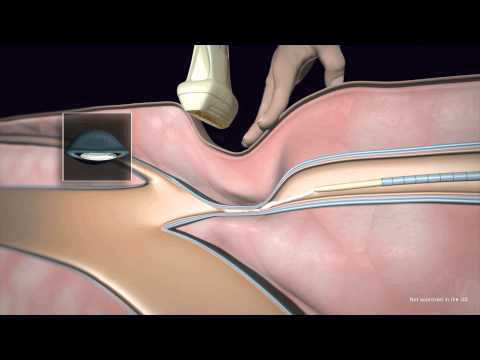
Medtronic’s VenaSeal closure system has been granted pre-market approval (PMA) from the US Food and Drug Administration (FDA) for the treatment of symptomatic venous reflux.
This minimally invasive procedure is the first and only non-tumescent, non-thermal, non-sclerosant procedure approved for...
Peter H Lin
Pulmonary embolism in children is a potentially lethal condition and yet is a vastly understudied arena. Autopsy studies show a higher prevalence of pulmonary embolism compared to medical database registries suggesting that this condition is often clinically...
Highlights: -"Working with radiation is like keeping a pet tiger in your living room" -Wearable exercise tracker improves intermittent claudication symptoms at six months -Endovascular revolution in the aorta: 25 years of a landmark case -Profile: Sebastian Debus -Rabih...
Highlights: -"Working with radiation is like keeping a pet tiger in your living room" -Wearable exercise tracker improves intermittent claudication symptoms at six months -Endovascular revolution in the aorta: 25 years of a landmark case -Profile: Sebastian Debus -Rabih...
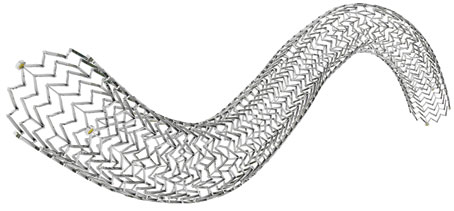
At procedure, Zilver Vena stent placement resulted in a more than two-fold improvement in the vessel minimum lumen diameter. Based on available follow-up data, stent placement has corresponded to improved clinical symptoms.
The City University of New York (CUNY), Lawrence Livermore National Laboratory, and Near Infrared Imaging, have released the “Vein-Eye” camera. The Vein-Eye provides enhanced visualisation of veins when drawing blood or placing IVs in a patient’s arm or hand....
By Johann Chris Ragg
Patients with saphenous insufficiency can undergo valve zone shaping instead of destructive methods by surgical removal or endovenous closure, says Johann Chris Ragg. He presented the new vein restoring modality at the European Venous Forum (EVF;...
Dedicated venous stents can be effectively used to relieve outflow obstruction at one year following thrombotic disease in both the acute and chronic setting, according to the prize-winning research presented at the European Venous Forum (EVF; 2–4 July, Saint...
The National Institute for Health and Care Excellence (NICE), the medicines cost-effectiveness body for England and Wales, has recommended a new treatment to help patients suffering from blood clots in the legs and lungs.NICE has issued its final recommendation...
The study concluded that treatment with ultrasound-facilitated catheter-directed low-dose thrombolysis for acute pulmonary embolism improves right heart function, reduces blood clot size, and decreases pulmonary hypertension in patients with intermediate to high risk pulmonary embolism.
The first patient has been enrolled in ETNA-AF-Europe (Edoxaban treatment in routine clinical practice – atrial fibrillation – Europe), and ETNA-VTE-Europe (Edoxaban treatment in routine clinical practice – venous thromboembolism – Europe) has commenced.
Daiichi Sankyo’s European ETNA registries are...
Results of the landmark CATCH (Comparison of Acute Treatments in Cancer Haemostasis) study were published in The Journal of American Medical Association (JAMA), comparing innohep (tinzaparin), a low-molecular weight heparin (LMWH), with warfarin in patients with cancer-associated thrombosis.
In the study, sponsored by...
BTG announced on 6 August 2015 that Health Canada has issued a notice of compliance approving polidocanol injectable foam (Varithena, BTG) for the treatment of incompetent great saphenous veins, accessory saphenous veins, and visible varicosities of the great saphenous...
By Samuel Money
Open vena caval surgery is rare. It is mainly performed for oncological indications when the inferior vena cava is involved by an adjacent tumour. One of the most common malignancies that affect the inferior vena cava is...
Ian Franklin, a member of the CX Programme Organising Board and director of the CX Venous Workshop, speaks about new technologies for the treatment of varicose veins and about how deep venous interventions have been incorporated to the CX...
After the European launch in April 2015, Biolas has introduced the VariClose Vein Sealing Systems in the European market for the treatment of varicose veins.
NICE has issued a Final Appraisal Determination (FAD) for Lixiana (edoxaban) for the treatment and prevention of recurrent deep vein thrombosis (DVT) and pulmonary embolism (PE) in adults.
BioGlue is now indicated for adhesion and support of haemostasis for aortotomy closure sites, suture/anastomosis sites (including aortic dissection and anastomosis sites with a prosthetic graft), and suture sites on the heart.
Twelve-month results from the VeClose trial have demonstrated cyanoacrylate adhesive (CAE) is non-inferior to radiofrequency ablation (RFA) for the treatment of incompetent great saphenous veins.
The 12-month closure rates for Medtronic's VenaSeal closure system are comparable to those achieved using radiofrequency ablation.
Highlights: -One-year IMPROVE data suggest benefit of EVAR in ruptured aneurysms -New Eluvia drug-eluting stent shows 94.4% primary patency rate at nine months -Michael Dake: Arch branded device -Andrew Holden: Bioresorbable stents -Profile: Janet Powell
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/66-Vascular-News-USA.pdf
Highlights: -One-year IMPROVE data suggest benefit of EVAR in ruptured aneurysms -New Eluvia drug-eluting stent shows 94.4% primary patency rate at nine months -Michael Dake: Arch branded device -Andrew Holden: Bioresorbable stents -Profile: Janet Powell
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/66-Vascular-News-EU.pdf
Jan Heyligers and Patrick Vriens, Tilburg, The Netherlands, write about a technique using the great saphenous vein for the reconstruction of an infected aorta. The technique, defined as a “see one, do one” procedure, was presented at the Charing...
The decision provides supplemental reimbursement to US hospitals for the new medical device with the potential to improve outcomes for patients undergoing a percutaneous transthoracic lung biopsy.
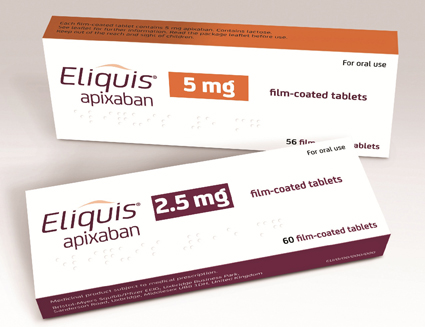
Bristol-Myers Squibb and Pfizer have announced that the National Institute for Health and Care Excellence (NICE) has published final guidance recommending the use of Eliquis (apixaban) as an option for the treatment of deep vein thrombosis (DVT) and pulmonary...
As part of a 90-day implementation plan, the NHS now has three months for apixaban to be made available to patients in England and Wales whose doctors believe it is a clinically appropriate treatment for them.
This video tells the story of one of the world’s most prominent heart surgeons, Edward Diethrich, and the career-altering health issues he has faced as a result of chronic, low-level exposure to ionising radiation through his work.
https://youtu.be/rXgt0bF3GJM
Highlights: -Discussion of controversies tops Charing Cross 2015 agenda -CX Venous Workshop -CX Live Peripheral Arterial Cases -CX Edited Live Cases -CX ilegx Collaboration Day -Summary of Vascular News and Interventional News profiles 2014
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/CX-Vascular-News-2015-US_low-res.pdf
The second day of the CX Venous Workshop was held yesterday on the Upper Level of the Gallery, rounding off a busy week in which more than 950 people visited over the two days—a new record for the Workshop....
Yesterday saw the beginning of the two-day CX Venous Workshop, in which delegates can enjoy expert demonstrations of a selection of the most interesting and important phlebological technologies currently in use. Now in its seventh year, the event continues...
Observational studies show a sharp increase in the placement of inferior vena cava filters over the past three decades, including their use as add-on therapy to anticoagulant therapy in patients presenting with a blood clot.
Daiichi Sankyo has announced that Swissmedic, the regulatory authority of Switzerland, has granted approval of Lixiana (edoxaban), an oral, once-daily selective factor Xa inhibitor, for the prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation. Simultaneously,...
Early data from a multicentre, single-arm study that set out to evaluate the safety and effectiveness of a novel approach to inferior vena cava filtration using the VenaTech convertible filter (B Braun Interventional Systems) suggest that the use of...
Intrinsic Imaging has been awarded a clinical trial to study an interventional medical device designed for the prevention of pulmonary emboli.
Throughout this trial, Intrinsic Imaging will provide imaging core lab services including, but not limited to, protocol and charter...
Highlights: -Discussion of controversies tops Charing Cross 2015 agenda -CX Venous Workshop -CX Live Peripheral Arterial Cases -CX Edited Live Cases -CX ilegx Collaboration Day -Summary of Vascular News and Interventional News profiles 2014
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/CX-Vascular-News-2015-EU_low-res.pdf
The Medtronic VenaSeal system is intended for patients with symptomatic superficial varicose veins of the legs.
biolas has announced the launch of its VariClose vein sealing systems in Europe.
VariClose is a new technique by which an incompetent saphenous vein is sealed through embolization. biolas says that the procedure is simpler and more successful than older...
Medtronic announced today that a previously communicated global voluntary recall to address an issue with certain lots of its Trellis-6 and Trellis-8 peripheral infusion systems has now been classified as a Class 1 recall by the US Food and...
The US Food and Drug Administration (FDA) has approved Daiichi Sankyo’s Savaysa (edoxaban), an oral, once-daily selective factor Xa inhibitor, to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation.
In ENGAGE AF-TIMI 48, Savaysa...
Highlights: -Publishing individual outcome data way make physicians risk averse -Early results suggest Lithoplasty is effective in the treatment of calcified lesions -SPACE 2 cartoid study is halted -Juan Perodi: A humble patient -Rocha-Singh: DEBs below-the-knee -Profile: Jean-Baptiste Ricco
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/65-Vascular-News-US_low-res-1.pdf
Highlights: -Publishing individual outcome data way make physicians risk averse -Early results suggest Lithoplasty is effective in the treatment of calcified lesions -SPACE 2 cartoid study is halted -Juan Perodi: A humble patient -Rocha-Singh: DEBs below-the-knee -Profile: Jean-Baptiste Ricco
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/65-Vascular-News-EU_low-res.pdf
Leaving the devices in place risks filter fracture or symptoms from penetration of filter components outside of the vein into adjacent structures, increased risk of new blood clots in the legs, and other complications.
By David Dudzinski
Transthoracic echocardiography offers real-time information that assists vascular specialists in the diagnostic and prognostic evaluation of acute pulmonary embolism. Specific roles of transthoracic echocardiography are subject to local practice and expertise as there is no formulaic approach...
By Ido Weinberg @Angiologist
The Merriam-Webster dictionary defines social media as “forms of electronic communication (as websites for social networking and microblogging) through which users create online communities to share information, ideas, personal messages, and other content (as videos).” So,...
Highlights: -C-arm angulation increases radiation exposure to operators during complex EVAR -EXCITE ISR results show superiority of laser atherectomy over angioplasty alone -Lutonix becomes first drug-eluting balloon to be approved in USA -Lindsay Machan: Reducing radiation -Ido Weinberg: Social...
Highlights: -C-arm angulation increases radiation exposure to operators during complex EVAR -EXCITE ISR results show superiority of laser atherectomy over angioplasty alone -Lutonix becomes first drug-eluting balloon to be approved in USA -Lindsay Machan: Reducing radiation -Ido Weinberg: Social...
Veniti has enrolled the first US patients in the VIRTUS trial of the Venti Vici venous stent system, with four procedures performed at two sites.
Cardinal Health has announced that its MynxGrip Vascular Closure Device recently received Food and Drug Administration (FDA) approval for use to close femoral veins. The MynxGrip device is now indicated for use to seal 5F, 6F and 7F femoral arterial and femoral venous access sites.
Highlights: -Two-stage TEVAR yields lower mortality and more protection against spinal cord injury -Endovascular repair of popliteal aneurysm maintains significant sac shrinkage at five years -Incraft low-profile endograft launched in Europe and Canada -Thomas Zeller: Drug-eluting balloons -Philip S...
Highlights: -Two-stage TEVAR yields lower mortality and more protection against spinal cord injury -Endovascular repair of popliteal aneurysm maintains significant sac shrinkage at five years -Incraft low-profile endograft launched in Europe and Canada -Thomas Zeller: Drug-eluting balloons -Philip S...
Novate Medical has announced that Souheil Saddekni, professor of Vascular and Interventional Radiology at the University of Alabama, Birmingham, USA, has enrolled the first patient in the SENTRY IDE study.
The Editors of the European Journal of Vascular and Endovascular Surgery have announced the introduction of a new Open Access, online journal to run alongside the existing journal.
Bristol-Myers Squibb and Pfizer have announced results of a pre-specified secondary analysis of the Eliquis phase 3 AMPLIFY-EXT trial (Apixaban after the initial management of pulmonary embolism and deep vein thrombosis with first-line therapy-extended treatment). The analysis evaluated clinical...
By Mark S Whiteley
Since the NICE (National Institute for Health and Care Excellence) guidelines for the treatment of varicose veins was published in July 2013, endovenous thermal ablation has now “come of age”. Recommended as the first line treatment...
BTG International has announced that the first varicose vein patient has been treated with Varithena (polidocanol injectable foam), the only FDA-approved foam for the treatment of incompetent great saphenous veins, accessory saphenous veins and visible varicosities of the great saphenous...
Bristol‐Myers Squibb Company and Pfizer have announced that the European Commission has approved Eliquis (apixaban) for the treatment of deep vein thrombosis and pulmonary embolism, and the prevention of recurrent deep vein thrombosis and pulmonary embolism in adults. The...
A study published in the Journal of the American Medical Association (JAMA) has found no difference in mortality rates between deep vein thrombosis patients treated with catheter-directed thrombolysis or anticoagulation alone. In the study, evidence of higher adverse events...
Veniti has announced that it has received approval from the United States Food and Drug Administration (FDA) for an Investigational Device Exemption (IDE) to begin the VIRTUS trial of the Vici venous stent system. The Vici venous stent system...
The Heart and Vascular Outcomes Research Institute (HVORI) is collaborating with a number of medical centres to launch the iRetrieve study aimed to improve the retrieval rate of inferior vena cava (IVC) filters. John E Rectenwald, associate professor of...
Covidien has announced the commercial launch of its next generation Trellis peripheral infusion system. The redesigned system continues to be the only pharmacomechanical thrombolysis device that enables focused treatment of blood clots that lead to post-thrombotic syndrome. This latest Trellis system has...
Highlights: -CX 2014 audience recognises the impact of drug-eluting balloons -No type I or III endoleaks with the Incraft system for EVAR at two years -Lindsay Machan: Radiation hazards -Mark S Whiteley: Heat ablation -Profile: Cliff Shearman
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/62-Vascular-News-USA_low-res.pdf
Highlights: -CX 2014 audience recognises the impact of drug-eluting balloons -No type I or III endoleaks with the Incraft system for EVAR at two years -Lindsay Machan: Radiation hazards -Mark S Whiteley: Heat ablation -Profile: Cliff Shearman
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/62-Vascular-News_low-res.pdf
Bristol-Myers Squibb Company and Pfizer have announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has adopted a positive opinion recommending that Eliquis (apixaban) be granted marketing authorisation for the treatment...
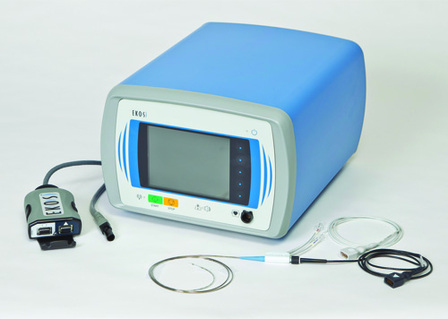
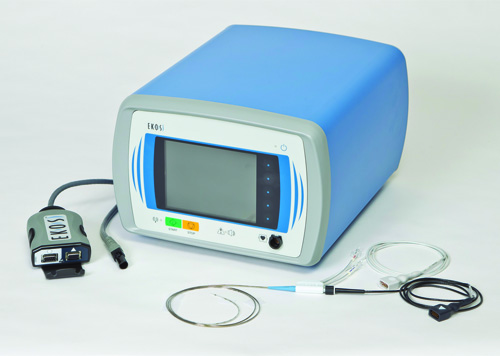
EKOS Corporation has announced the start of patient enrolment for the Accelerated thrombolysis for post-thrombotic syndrome using the EkoSonic endovascular system (ACCESS PTS) study.
The ACCESS PTS study is a prospective, single-arm, multicentre trial, designed to evaluate the safety and...
Boehringer Ingelheim has announced that Pradaxa (dabigatran etexilate) has been approved by the European Commission for the treatment and prevention of recurrence of deep vein thrombosis and pulmonary embolism. The US Food and Drug Administration (FDA) approved Pradaxa for...
Cook Medical is engaged in two clinical studies that will provide additional data on the safety and effectiveness of inferior vena cava filters.
The first study, the Cook Inferior Vena Cava Filter (CIVC) study, will add to Cook’s existing clinical...
Women with significant pelvic vein incompetence are likely to have haemorrhoids, which could suggest a causal link, according to research from a varicose vein clinic.
Judy Holdstock (Whiteley Clinic, London) told the Charing Cross Symposium (5–8 April; London, UK) that...
Ekos Corporation has announced that the US Food and Drug Administration (FDA) has cleared the EkoSonic endovascular system for the ultrasound facilitated, controlled and selective infusion of physician-specified fluids, including thrombolytics, into the vasculature for the treatment of pulmonary embolism.
Stephen Black, St George’s Hospital, London, UK, told delegates at the Charing Cross Symposium (5–8 April 2014, London, UK) that most of the evidence for deep venous reconstruction is based on single-centre experiences, many of them small, and therefore...
Boehringer Ingelheim Pharmaceuticals announced that the US Food and Drug Administration (FDA) has approved Pradaxa (dabigatran etexilate mesylate) for the treatment of deep venous thrombosis and pulmonary embolism in patients who have been treated with a parenteral anticoagulant for...
At the CX Office-based Vein Practice Course, delegates were granted the unique opportunity to view demonstrations and try office-based venous devices and procedures with the guidance of experienced tutors. The 2014 CX Office-based Practice Course saw record attendance with...
Daiichi Sankyo announced late-breaking data from two pre-specified subgroup analyses of East Asian patients with non-valvular atrial fibrillation or venous thromboembolism enrolled in two phase 3 edoxaban studies. The findings of the two subgroup analyses of 1,943 East Asian...
Recognising that new technologies and techniques are revolutionising how venous disorders are treated, in 2014, for the first time, the Charing Cross Symposium (5–8 April, London, UK) will host three days of venous activities. This year, the CX Office-based...
Highlights: -No difference between endovascular and open repair for ruptured aneurysms -Renal artery stenting no better than medical therapy alone in CORAL -Hybrid techniques for the arch: are they effective and durable? By Piergiorgio Cao -Profile: Andrew Holden
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/61-Vascular-News_low-res_USA.pdf
Highlights: -No difference between endovascular and open repair for ruptured aneurysms -Renal artery stenting no better than medical therapy alone in CORAL-Hybrid techniques for the arch: are they effective and durable? By Piergiorgio Cao-Profile: Andrew Holden
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/61-Vascular-News_low-res.pdf
Veniti announced on 23 January 2014 that patients in the European Union (EU) were successfully treated for symptomatic venous outflow obstruction of the lower extremities with the Veniti Vici Venous Stent System. These procedures mark the first uses in...
EKOS Corporation announced on 20 January 2014 the publication of the results of its ULTIMA (Ultrasound accelerated thrombolysis of pulmonary embolism) trial in Circulation. The announcement was made at the International Symposium on Endovascular Therapy (ISET) in Miami, USA.
The ULTIMA...
Argon Medical Devices announced that it has received clearance from the US Food and Drug Administration to begin marketing the Option Elite retrievable inferior vena cava filter with a new over-the-wire delivery technique on 16 January 2013.
According to the...
Sapheon has announced that it has submitted the second module of the pre-market approval (PMA) application for the VenaSeal Sapheon Closure System to the US Food and Drug Administration (FDA). According to the company, the submission, which was completed...
Daiichi Sankyo is seeking approval in Japan for edoxaban in new indications for non-valvular atrial fibrillation and symptomatic venous thromboembolism.
Daiichi Sankyo has announced that it has submitted a supplemental new drug application for its oral, once-daily direct factor Xa-inhibitor edoxaban (Lixiana)...
Boehringer Ingelheim has announced results from the RE-COVER II study evaluating dabigatran compared to warfarin in patients diagnosed with acute deep vein thrombosis and/or pulmonary embolism. In this phase III study, published online in the American Heart Association’s journal...
Lowell S Kabnick, associate professor, NYU Langone Medical Center, New York, USA, has worked to develop minimally invasive systems for the treatment of varicose veins. His proudest moment was the development of the TreSheath device, which carries the laser...
Highlights: -Vascular surgery societies refuse to endorse TASC III -ESVS announces bankruptcy of annual meeting organiser -3D contrast-enhanced ultrasound potentially better at detecting endoleaks -Profile: Lowell Kabnick
Highlights: -Vascular surgery societies refuse to endorse TASC III -ESVS announces bankruptcy of annual meeting organiser -3D contrast-enhanced ultrasound potentially better at detecting endoleaks -Profile: Lowell Kabnick
BTG has announced that the FDA has approved polidocanol injectable foam (Varithena) for the treatment of patients with incompetent veins and visible varicosities of the great saphenous vein system.
Varithena (formerly known as Varisolve PEM) is a pharmaceutical-grade, low-nitrogen, polidocanol...
Highlights: -Vascular surgery societies refuse to endorse TASC III -ESVS announces bankruptcy of annual meeting organiser -3D contrast-enhanced ultrasound potentially better at detecting endoleaks -Profile: Lowell Kabnick
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/60-Vascular-News_US.pdf
Highlights: -Vascular surgery societies refuse to endorse TASC III -ESVS announces bankruptcy of annual meeting organiser -3D contrast-enhanced ultrasound potentially better at detecting endoleaks -Profile: Lowell Kabnick
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/60-Vascular-News_low-res.pdf
The company's Limerick site develops peripheral vascular, gastroenterology and urology devices for global distribution, and the Innovation Centre is a dedicated space enabling the company to collaborate closely with physicians.
The double helical, symmetrical design of the Crux device helps prevent filter tilt, as the helical design self-centres automatically within the vena cava upon deployment. The device offers the Bi-Trieval option of retrieval via either the jugular or femoral vein.
By Brian G DeRubertis
Surgical caval interruption for prevention of fatal pulmonary embolisation had been performed since the 1950s, though the modern era of pulmonary embolism prevention began with the introduction of the implantable inferior vena cava filters. Initial experience...
A new rotational thrombectomy device is safe and effective in the treatment of acute and subacute deep vein thrombosis in a single session of pharmacomechanical thrombolysis providing results of improved functional outcome.
The results of a multicentre registry with the...
Researchers from Universite Laval’s Faculty of Medicine and CHU de Quebec have shown that it is possible to treat venous ulcers unresponsive to conventional treatment with wound dressings made from human skin grown in vitro. A study published in...
A UK national initiative to carry out mandatory screening of hospital patients for deep vein thrombosis has resulted in a “significant” reduction in death rates, experts in Birmingham have concluded. A major study was carried out involving every single patient admitted...
Highlights: -Inadequate sealing is top reason for endograft explant -"Aortic debranching safe and durable up to five years" -A unified voice for vascular surgery in Latin America by Alberto Munoz -Profile: Thomas Zeller
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/59-Vascular-News_USA.pdf
Highlights: -Inadequate sealing is top reason for endograft explant -"Aortic debranching safe and durable up to five years" -A unified voice for vascular surgery in Latin America by Alberto Munoz -Profile: Thomas Zeller
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/59-Vascular-News_low-res.pdf
Highlights: -Inadequate sealing is top reason for endograft explant -"Aortic debranching safe and durable up to five years" -A unified voice for vascular surgery in Latin America by Alberto Munoz -Profile: Thomas Zeller.
Highlights: -Inadequate sealing is top reason for endograft explant -"Aortic debranching safe and durable up to five years" -A unified voice for vascular surgery in Latin America by Alberto Munoz -Profile: Thomas Zeller.
LeMaitre Vascular has announced that it acquired the assets of InaVein for US$2.5mm, or 1.1X InaVein’s 2012 sales, and potential earn-out payments in 2014 and 2015 based on the performance of the acquired business and regulatory approval in China....
Results from the Hokusai-VTE study presented at the European Society of Cardiology (ESC) congress have shown that the oral anticoagulant edoxaban was non-inferior to warfarin in the treatment of venous thromboembolism (VTE). The randomised trial also demonstrated that the...
Sapheon announced on 9 September 2013 that it has completed enrolment in the US pivotal study of the VenaSeal Sapheon Closure System. According to the company, 242 patients have been enrolled in the study.
VenaSeal is a minimally invasive, single...
Tactile Medical has launched an ambulatory device for patients with chronic wounds. The ACTitouch Adaptive Compression Therapy system combines two proven therapies—intermittent and sustained compression—in one device to heal venous leg ulcers.
Sustained compression has long been the standard of...
There is no evidence that impaired blood flow or blockage in the veins of the neck or head is involved in multiple sclerosis, says a study from McMaster University.
The research, published online by PLOS ONE on 14 August 2013, found no evidence...
Haroun Gajraj, director of the The VeinCare Centre, Bristol, UK, speaks about using medical super glue for the treatment of varicose veins and superficial venous reflux.
Thermocoagulation with a new endovenous radiofrequency system is a safe and efficient technique in the treatment of reflux of the great saphenous vein, according to a pilot study conducted in Belgium.
Thermocoagulation with EVRF (F Care Systems) is a treatment...
Surgery should only be offered to treat varicose veins if other less invasive treatments are unsuitable for patients, according to a guidance issued by the UK National Institute for Health and Care Excellence (NICE) on 24 July.
For the first...
A multicentre study has confirmed the effectiveness of intermittent pneumatic compression systems in reducing the incidence of deep vein thrombosis following a stroke. According to a press release, incidence could be reduced by 29%.
A review of the literature suggests there is insufficient evidence to support the use of intravascular filters or augmented dosing of anticlotting medication in patients undergoing bariatric surgery to prevent venous thromboembolism, according to a report published online first...
EKOS owns, manufactures and distributes the EkoSonic Endovascular System (EkoSonic), an interventional device that uses a locoregional approach in the treatment of severe blood clots.
Highlights: - Management of type II endoleak divides the experts at CX35 - Intermittent claudication: Lack of funding for supervised exercise programmes remains a global problem - First-in-man implantation of left subclavian artery branched TEVAR device
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/58-Vascular-News-US_low-res.pdf
Highlights: - Management of type II endoleak divides the experts at CX35 - Intermittent claudication: Lack of funding for supervised exercise programmes remains a global problem - First-in-man implantation of left subclavian artery branched TEVAR device
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/58-Vascular-News.pdf
In the US study with the device, there was 100% freedom from aneurysm-related mortality, 0% post-implant aneurysm rupture, 0% graft migration and 0% conversion to open repair for the 107 patients followed to three years.
Highlights: - Management of type II endoleak divides the experts at CX35 - Intermittent claudication: Lack of funding for supervised exercise programmes remains a global problem - First-in-man implantation of left subclavian artery branched TEVAR device
Highlights:
- Management of type II endoleak divides the experts at CX35
- Intermittent claudication: Lack of funding for supervised exercise programmes remains a global problem
- First-in-man implantation of left subclavian artery branched TEVAR device
At CX35 (6–9 April, London, UK), Cees Wittens, Maastricht, The Netherlands, presented his experience with stents for deep vein occlusive disease. He told delegates that the ideal device should offer high radial force and high flexibility.
Wittens stated that stenting...
Gerard Stansby, Newcastle, UK, told delegates at CX35 (6–9 April 2013, London, UK) that the use of glue in varicose veins in safe and effective and that, if it continues to show good results, it will be a very...
The UK National Institute for Health and Care Excellence (NICE) has issued final draft guidance recommending rivaroxaban (Xarelto, Bayer Healthcare) as a clinically and cost-effective option for treating pulmonary embolism and preventing recurrent deep vein thrombosis and pulmonary embolism...
Cook Medical has launched the VIVO clinical research study to evaluate the safety and effectiveness of the Zilver Vena Venous Self-Expanding Stent in the treatment of symptomatic iliofemoral venous outflow obstruction. This disease is characterised by leg pain, throbbing,...
Highlights:
-Clinical need drives intraoperative imaging to the next level
-CX ilegx Collaboration embraces Electronic Endovascular Education
-A completely percutaneous closure approach is feasible in most cases
-Hands-on training and learning at the CX Office-Based Vein Practice Course
Highlights:
-Experts sharply divided on type II endoleak challenge
-Cordis launches Smart Flex stent at CX35
-Developments in imaging go under the scanner at CX35
-CX St George's Vascular Access Course explores new technologies and research
Highlights:
-Intermittent claudication - Lack of funding for supervised exercise programmes is a global problem
-Latin America comes to CX
-Latest data from peripheral trials presented at CX35
-Substantial improvement in functional status with bioresorbable scaffold at 30 days
Gerard O'Sullivan, Galway, Ireland, CX35 delegates that computed tomography pulmonary angiography (CTPA) and CT venography are his preferred imaging modalities.
Results of the ULTIMA pulmonary embolism trial comparing endovascular therapy to standard of care were presented at the American College of Cardiology meeting in San Francisco, USA, by Nils Kucher, University Hospital, Bern, Switzerland.
ULTIMA is the world’s first randomised...
Highlights:
-What to expect from CX35
-Advanced imaging technology at the heart of CX35
-CX35 provides record number of educational opportunities
-Summary of Vascular News and Interventional News’ profiles 2012
The New England Journal of Medicine has published findings from the RE-MEDY and RE-SONATE trials investigating dabigatran etexilate (Pradaxa, Boehringer Ingelheim) in the long-term prevention of deep vein thrombosis (DVT) or pulmonary embolism (PE). The results demonstrate that Pradaxa 150mg...
By Mohsen Sharifi
Percutaneous endovenous intervention (PEVI) is increasingly being used in the treatment of massive lower extremity deep vein thrombosis. It utilises a variety of treatment modalities including catheter-directed thrombolysis, various thrombectomy devices, balloon venoplasty and stenting. There is...
ALN (ALN Implants Chirurgicaux, France) announced the launching in the USA of its new ALN Vena Cava Filter with Hook, which recently received the CE mark and Food and Drug Administration (FDA) approval.
The ALN Vena Cava Filter with Hook...
By Russell Samson
Aspirin finds a role again
Before the advent of more potent heparin, low molecular weight heparins and warfarin, aspirin was used extensively in the treatment and prevention of deep vein thrombophlebitis. However, these medications have largely replaced aspirin. Recently,...
Daiichi Sankyo Europe announced the enrolment of the first patient into the PREFER in VTE (Prevention of thromboembolic events – European registry in venous thromboembolism) study on 6 February 2013. PREFER in VTE is the first patient registry to...
Highlights:-Initial experience of total endovascular arch aneurysm exclusion is "encouraging"-Stroke associated with a threefold increased future mortality in CREST-Advances in drug therapy for the treatment of thrombophlebitis and pulmonary embolism-Profile: Peter Gloviczki
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/57-Vascular-News_USA_low-res.pdf
Highlights: -Initial experience of total endovascular arch aneurysm exclusion is "encouraging" -Stroke associated with a threefold increased future mortality in CREST -Advances in drug therapy for the treatment of thrombophlebitis and pulmonary embolism -Profile: Peter Gloviczki
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/57-Vascular-News_low-res.pdf
Peter Gloviczki, professor of Surgery, College of Medicine, Mayo Clinic, Rochester, USA, is the president of the Society for Vascular Surgery (SVS) 2012–2013. Gloviczki, who started his career in Hungary, moved to the United States in 1981 to work...
Highlights:-Initial experience of total endovascular arch aneurysm exclusion is "encouraging"-Stroke associated with a threefold increased future mortality in CREST-Advances in drug therapy for the treatment of thrombophlebitis and pulmonary embolism-Profile: Peter Gloviczki
Highlights:-Initial experience of total endovascular arch aneurysm exclusion is "encouraging"-Stroke associated with a threefold increased future mortality in CREST-Advances in drug therapy for the treatment of thrombophlebitis and pulmonary embolism-Profile: Peter Gloviczki
The results of PEARL (Registry of AngioJet use in the peripheral vascular system), a phase II multicentre registry, led by Robert Lookstein, chief of interventional radiology, Mount Sinai Medical Center, New York, USA, have suggested that rheolytic pharmacomechanical thrombectomy...
Covidien announced the five-year results of the ClosureFast Long-Term European Multi-Center Study in patients with chronic venous insufficiency in December. The ClosureFast study was conducted prospectively at eight centres in Europe. The Venefit procedure (using the ClosureFast radiofrequency ablation...
On 3 December, Volcano Corporation announced it has signed an agreement to acquire Crux Biomedical, developer of the Crux VCF System―an inferior vena cava (IVC) filter designed to prevent pulmonary embolisms.The Crux VCF System, which is designed to facilitate bi-directional...
Highlights: -Early follow-up shows promising results with bioabsorbable stent in the superficial femoral artery -Is the Cotavance withdrawal in the USA a setback for drug-eluting balloons? -ACST-2 first results indicate carotid revascularisation is becoming safer -Profile: Piergiorgio Cao
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/56-Vascular-News-USA_low-res.pdf
Highlights: -Early follow-up shows promising results with bioabsorbable stent in the superficial femoral artery -Is the Cotavance withdrawal in the USA a setback for drug-eluting balloons? -ACST-2 first results indicate carotid revascularisation is becoming safer -Profile: Piergiorgio Cao
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/56-Vascular-News_low-res.pdf
Rivaroxaban (Xarelto, Bayer HealthCare) has been approved by the European Commission for the treatment of pulmonary embolism (PE) and the prevention of recurrent deep vein thrombosis (DVT) and PE in adults. This approval makes rivaroxaban the only novel oral...
Highlights:
-Early follow-up shows promising results with bioabsorbable stent in the superficial femoral artery
-Is the Cotavance withdrawal in the USA a setback for drug-eluting balloons?
-ACST-2 first results indicate carotid revascularisation is becoming safer
-Profile: Piergiorgio Cao
Highlights:
-Early follow-up shows promising results with bioabsorbable stent in the superficial femoral artery
-Is the Cotavance withdrawal in the USA a setback for drug-eluting balloons?
-ACST-2 first results indicate carotid revascularisation is becoming safer
-Profile: Piergiorgio Cao
Low-dose aspirin prevents recurrent venous thromboembolism and major vascular events in patients with first unprovoked venous thromboembolism, results from the ASPIRE randomised controlled trial, presented at the American Heart Association Scientific Sessions (3–7 November, Los Angeles, USA) have shown....
On 2 November, the US Food and Drug Administration (FDA) expanded the approved use of rivaroxaban (Xarelto) to include treating deep vein thrombosis (DVT) or pulmonary embolism (PE), and to reduce the risk of recurrent DVT and PE following...
The European Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency has recommended the approval of the oral anticoagulant rivaroxaban for the treatment of pulmonary embolism and prevention of recurrent pulmonary embolism and deep vein...
A computerised checklist system designed to help physicians identify and use the best methods of preventing potentially venous thromboembolism (VTE) in hospitalised trauma patients dramatically reduced the number of these dangerous VTEs, according to researchers from the Johns Hopkins...
Highlights:-Intensive endovascular simulation training improves performance-Stem cell mobilisation shows trend in improving amputation rate in critical limb ischaemia-Chimney and periscope grafts safe and effective to facilitate EVAR or hybrid procedures for complex aortic aneurysms-Profile: Frank Lederle
Highlights:-Intensive endovascular simulation training improves performance-Stem cell mobilisation shows trend in improving amputation rate in critical limb ischaemia-Chimney and periscope grafts safe and effective to facilitate EVAR or hybrid procedures for complex aortic aneurysms-Profile: Frank Lederle
Following Health Canada approval, Cook Medical announced the Canadian launch of the Zilver Vena Venous Self-Expanding Stent at the 2012 Annual Meeting of the Canadian Society for Vascular Surgery. The Zilver Vena is designed specifically for stenting obstructed iliofemoral...
On 3 October, the UK’ National Institute for Health and Clinical Excellence (NICE) opened a consultation on a new draft quality standard for the management of venous thromboembolic (VTE) diseases. The draft quality standard describes markers of high-quality, cost-effective...
Highlights: -Intensive endovascular simulation training improves performance -Stem cell mobilisation shows trend in improving amputation rate in critical limb ischaemia -Chimney and periscope grafts safe and effective to facilitate EVAR or hybrid procedures for complex aortic aneurysms -Profile: Frank...
By David L Gillespie
Varicose veins are a common condition affecting more than 25 million people in the United States. While the genetic basis of varicose veins remains to be elucidated, it is clear they can arise in young individuals with...
Vascular Insights has announced the start of a multicentre randomised clinical trial conducted by principal investigators Alun H Davies, professor of Vascular Surgery and Ian J Franklin, consultant vascular surgeon, Imperial College London and Imperial College Healthcare NHS Trust.The...
Despite previous studies suggesting the contrary, statins may not prevent venous thrombo-embolism in adults, according to a large analysis by international researchers published recently in PLOS Medicine.
On 11 September 2012, Healthpoint Biotherapeutics announced the initiation of a phase III clinical trial investigating the efficacy of HP802-247 for the treatment of venous leg ulcers. HP802-247 is an investigational allogeneic living cell bioformulation containing keratinocytes and fibroblasts....
The new Crux inferior vena cava filter can be implanted and retrieved safely and shows a low rate of pulmonary embolism, Anthony J Comerota, director, Jobst Vascular Institute, adjunct professor of Surgery, University of Michigan, USA, told delegates at...
Sapheon announced one year data in a 38-patient clinical study of the VenaSeal Sapheon Closure System, an advanced approach to the treatment of saphenous vein disease based on a proprietary medical adhesive.All patients were treated without the use of...
Endovenous radiofrequency ablation is effective in the treatment of large diameter varicose veins irrespective of anatomical structure, according to a study presented by Andrey Petukhov, Research Institute of Experimental Medicine, Saint Petersburg, Russia, at the 13th Meeting of the...
Emily A Wood, Division of Vascular Surgery, Stony Brook University Medical Center, USA, spoke at the European Venous Forum Annual Meeting (28–30 June, Florence, Italy) on the results of a study that set out to quantify the incidence of...
The use of a new fibre for endovenous laser ablation of the great saphenous vein results in equal occlusion rates as achieved with a bare fibre, a new study shows. According to data presented at European Venous Forum (Florence,...
The US Food and Drug Administration (FDA) has cleared Crux Biomedical’s inferior vena cava filter with bi-directional retrieval for the prevention of pulmonary embolism. This feature allows retrieval of the device from the femoral or jugular veins, a key...
The use of ultrasound-accelerated catheter-directed thrombolysis to treat acute iliofemoral deep vein thrombosis continues to be feasible and safe, according to a study presented by Rob H W Strijkers (Department of Vascular Surgery and Cardiovascular Research Institute Masstricht, Maastricht...
The US Food and Drug Administration (FDA) has assigned a priority review designation to the supplemental new drug applications (sNDAs) filed on 2 May 2012 for rivaroxaban (Xarelto, Bayer/Janssen), an oral anticoagulant, seeking new indications to treat patients with...
Highlights: -Is the task for TASC III consensus too great? -World's first experience with a bioabsorbable stent for the superficial femoral artery presented at CX -Gene linked to abdominal aortic aneurysms is found -Profile: Ross Naylor
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/54-Vascular-News_low-res.pdf
On 27 June 2012, Covidien announced the launch of its “1 in 1,000” campaign to help educate women about the risk of pregnancy-related blood clots, one of the leading causes of maternal mortality in the developed world. The announcement...
New guidance published on 27 June 2012 by the UK National Institute for Health and Clinical Excellence (NICE) aims to reduce the current high toll of long-term ill health or death caused by venous thromboembolic diseases by clarifying for...
At two years, mechanochemical ablation (MOCA) is as efficacious as current endothermal techniques without the need of tumescent anaesthesia and is more effective than reported results of foam sclerotherapy of the great saphenous vein. Steve Elias, who presented the...
BiO2 Medical has announced that it has received CE mark approval for the Angel Catheter, a nitinol inferior vena cava (IVC) filter, permanently attached to a central venous catheter (CVC) for the use of preventing pulmonary embolism in critically...
Michael Gough, Leeds General Infirmary, Leeds, UK, presented long-term follow-up after endovenous laser ablation for great saphenous varicose veins at the 34th Charing Cross International Symposium in London, UK. The study reviewed a group of 63 patients (79 limbs)...
SEATTLE I and II are intended to further establish the safety and efficacy of ultrasound accelerated thrombolysis for treatment of pulmonary embolism.
On 16 March 2012, the Parliament gave statutory approval recognising vascular surgery as a specialty independent from general surgery in the United Kingdom.
Stryker’s Sustainability Solutions division has announced the launch of Restep, a compression sleeve for deep vein thrombosis treatment. Restep provides hospitals with a single-source opportunity for deep vein thrombosis compression sleeves and allows hospitals an opportunity to lower costs by...
Final guidance from the UK’s National Institute for Health and Clinical Excellence (NICE), released on 28 March, encourages further research into percutaneous venoplasty, a procedure which is claimed to relieve symptoms for some people with multiple sclerosis.The procedure aims...
Results of the EINSTEIN-PE study have shown that the oral anticoagulant rivaroxaban (Xarelto, Janssen and Bayer Healthcare) was comparable to today’s standard of care in treating patients with acute symptomatic pulmonary embolism and in preventing development of venous thromboembolism....
Mohsen Sharifi, adjunct associate professor of Medicine at A T Still University, Mesa, USA, presented new data demonstrating that percutaneous endovenous intervention (PEVI) is an alternative in the treatment of upper extremity deep vein thrombosis at the iCON meeting...
The majority of recurrences following thermal ablation for varicose veins are associated with perforating veins, results of the REVATA study have shown. The investigation involved 164 varicose vein patients who were treated with radiofrequency or endovenous laser ablation.The results...
Guering Eid-Lidt, Department of Interventional Cardiology, Instituto Nacional de Cardiología Ignacio Chávez, Mexico City, Mexico, spoke about long-term outcomes of percutaneous mechanical thrombectomy for severe pulmonary embolism at the iCON meeting in Arizona, USA.Eid-Lidt said that percutaneous mechanical thrombectomy...
The recommendations would authorise the FDA to collect US$595 million in user fees over five years, plus adjustments for inflation.
The US Food and Drug Administration FDA has granted 510(k) clearance to AngioDynamics to market its NeverTouch Direct Procedure Kit for use with the VenaCure EVLT Laser Vein Ablation System (AngioDynamics). The NeverTouch Direct offers physicians the ability to treat...
The anticoagulant rivaroxaban (Bayer HealthCare, Xarelto) has been accepted by the Scottish Medicines Consortium (SMC) for use in eligible patients within NHS Scotland in two new therapeutic indications, based on licences granted by the European Commission:
The prevention of stroke...
Vascular Solutions announced that it is marketing a reprocessing service for the ClosureFAST radiofrequency ablation catheter in the United States. The ClosureFAST catheter, which is manufactured and marketed by VNUS Medical Technologies, is widely used for performing endovenous therapy...
Highlights: -Are "off-the-shelf" fenestrated endografts seeing a new dawn? -Lower restenosis and re-occlusion rates with drug-eluting balloons compared to angioplasty in DEBATE-BTK -Dismantling the different myths around the CREST trial -Profile: Patrick Peeters
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/53-Vascular-News_low-res.pdf
By Jörn Oliver Balzer
Pulmonary embolism continues to be a major cause of morbidity and mortality in the United States. In most clinical situations, anticoagulation is the preferred form of therapy. The efficacy of inferior vena cava filters is still...
Crux Biomedical announced it has received CE mark approval for their inferior vena cava filter (IVCF) with bi-directional retrieval (BDR). The Crux Biomedical IVCF was designed to address the limitations of currently available vena cava filters including perforation, migration and...
Sapheon announced one year results in its first in-man safety trial of the Sapheon Closure system. At the one year mark, 100% of the great saphenous vein segments treated with this vein sealant remained completely closed by ultrasound criteria....
The Centers for Medicare and Medicaid Services (CMS) has issued proposed regulatory guidance for implementing the Physician Payments Sunshine Act developed by senators Herb Kohl and Chuck Grassley in the USA.
Researchers from Norway have found that additional treatment with catheter-directed thrombolysis (CDT), when compared to standard treatment of oral anticoagulation therapy and elastic compression stockings, reduces the risk of post-thrombotic syndrome (PTS) in patients who suffer from deep-vein thrombosis...
Bluegrass Vascular Technologies has announced patient enrolment in the first clinical study of its Surfacer Inside-Out access catheter system, a proprietary system that allows physicians to perform a novel “inside-out” approach to gain venous access. The prospective single-centre feasibility study...
On 12 December, Daiichi Sankyo announced the results of a pooled analysis showing that edoxaban, a direct oral once-daily Factor Xa inhibitor, significantly reduced the risk of developing venous thromboembolism (VTE) following total knee or hip arthroplasty, when compared...
David Rosenthal, clinical professor of Surgery at the Medical College of Georgia, and chief of Vascular Surgery, Atlanta Medical Center, Atlanta, USA, presented data on a novel, bioconvertible vena cava filter at the VEITHsymposium in New York.The new inferior...
Charles McCollum, University of Manchester, UK, became consultant and senior lecturer in Surgery at the Charing Cross Hospital, London, UK, in 1983, aged 32. At 38, he was appointed professor of Surgery in Manchester. In this interview with Vascular...
Highlights: -First-in-man experience heralds the era of bioabsorbable stents -Initial experience of total endovascular arch repair reported -Will long-term trial results boost endovascular treatment in the superficial femoral artery? -Profile: Charles McCollum
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/52-Vascular-News_low-res.pdf
https://youtu.be/ac_om5HCjvg
The US Food and Drug Administration FDA has issued a draft guidance aimed at fostering early-stage development of medical devices within the United States.
Simon Parvin, consultant vascular surgeon, secretary general of The European Society for Vascular Surgery (ESVS) speaks on the history and major achievements of the ESVS.
Highlights: -Physician-modified endografts provide alternative for inoperable juxtarenal aneurysms -Vascular surgeons achieve best reported outcomes after carotid endarterectomy in CREST -SVS presents Lifetime Achievement Award to Wesley S Moore
-Profile: Giancarlo Biamino
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/51-Vascular-News_low-res.pdf
Highlights: -Are the EVAR boundaries being possibly pushed too far? -TASC guidelines set to recommend "endovascular first" for all lessions -64% of CX delegates in favour of office-based vein practice -Profile: Barry Katzen
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/50-Vascular-News_low-res.pdf
Boehringer Ingelheim launched Actilyse Cathflo 2mg, a low-dose vial of the thrombolytic alteplase. Actilyse Cathflo 2mg is used to restore the patency of central venous access devices, including those used in haemodialysis.
The American Heart Association's scientific statement offers advice for identifying and treating people with massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension.
This novel device is intended for single patient use for the treatment of superficial varicose veins and varicosities associated with superficial reflux and incompetence of the great saphenous vein in the lower limbs.
Highlights: -Setback for gene therapy in TAMARIS -New EVAR devices presented at the VEITHsymposium -Is there a common theme for endovenous thermal ablation failure? -Profile: Anthony Comerota
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/49-Vascular-News_low-res.pdf
The EkoSonic system, originally designed to dissolve blood clots in arms and legs, is the new alternative for the treatment of pulmonary embolism; until now, drugs were the only approved treatment.
Highlights: - One-year results show DESTINY of drug elution below the knee - ACE trialists conclude that EVAR offer no benefit over open repair at five years - Two new lower profile EVAR devices launched at ESVS - Drug...
Highlights: - Europe set for wave of next generation EVAR devices - Abbott Vascular seeks to expand carotid stenting indications after CREST trial - Russell Samson: Conflict of interest - Profile: Carlo Setacci
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/47-Vascular-News_low-res.pdf
Highlights: -Landmark EVAR trials deliver new messages at Charing Cross -Future of renal artery stenting in peril -Differeing views on CREST -CX Office Based Veins Practice -St George's Vascular Access Course at CX
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/46-Vascular-News_low-res.pdf
The DUET study, a randomised trial conducted to compare standard catheter-directed thrombolysis vs. ultrasound-accelerated thrombolysis in The Netherlands, has begun enrolment of its 60 thrombo-embolic infra-inguinal disease patients.
Highlights: -Is the DESTINY of drug-eluting stents below the knee? -European renal artery stenting trials draw fire from New York -Veith reports EVAR is superior to open repair for ruptured aneurysms -Profile: Peter Taylor
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/45-Vascular-Europe_lowres.pdf
Highlights: -ASTRAL trial results show no benefit for renal artery stenting -Cutting balloon no better than angioplasty -Setback for drug elution in the periphery as STRIDES follows SIROCCO -Profile: Roy Greenberg
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/44-Vascular-Europe_low-res.pdf
Highlights: -ICSS concludes carotid surgery is safer than stenting -Re-intervention higher after endovascular repair -Practice makes perfect for lower limb intervention -Profile: Michael Jacobs
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/43-Vascular-News_lowres.pdf
Minimally invasive treatment for varicose veins to be showcased at National Health Service EXPO
Highlights: -NICE UK concludes that EVAR is cost-effective -Endovascular aortic questions to be settled by individual patient data meta-analysis -Kabnick: Limitations of the RECOVERY trial -Pedrini: TASC II guidelines are little help -Profile: John Mannick
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/42-Vascular-News_lowres.pdf
UK NHS National Innovation Centre identifies the VNUS Closure procedure as a "select innovative technology"
Here is a personal view by Roger Greenhalgh about the management of varicose veins. It comes from a vascular surgeon who has spent most of his operating life working on arterial reconstruction, and, latterly, with the endovascular opportunities to...
Dr Nick Morrison will open the CX Venous Day, on Tuesday 7 April, with a talk on foam sclerotherapy.
Highlights: -MIMIC proves benefit of angioplasty for claudicants -DREAM results shake New York -Swiss show superior foot care -Profile: Michael Gough
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/41-Vascular-News_lowres.pdf
No significant difference between silver-donating and low-adherent dressings was found in a trial 213 patients (107 randomised to silver, 106 to low-adherent control), presented at the Vascular Society of Great Britain and Ireland meeting. Ulcer healing was...
Highlights: -UK NICE recommendations positive for EVAR -BASIL trial bypass benefit debated -Final ACST results: Sustained for early surgery -Profile: Michael DeBakey 1908-2008
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/40-Vascular-News_lowres.pdf
Highlights: -Carotid trials reappraised -First ruptured thoracic aneurysm trial supports TEVAR -Profile: George Hamilton
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/39-Vascular-News_lowres.pdf
0
DVT, other than that triggered by trauma or surgery, is now considered to be a chronic disease with a high risk of recurrence.
"ClosureFAST provides the ability to deliver high-energy dosage to obtain a successful treatment ..."
Results at the Society of Interventional Radiology's 32nd Annual Scientific Meeting, in Seattle, WA, Dr Mark J Garcia.
Tuesday 17th April at the Charing Cross Symposium
A fascinating exchange of views at this year's VEITHsymposium, three experts on venous disease presented data on various treatments for varicose veins.
Professor Roger Greenhalgh pays a fitting tribute to Professor Edmondo Malan at this year's IVEC 2006 meeting.
Stephen Greenhalgh, London, UK, presented data in November 2006 at the Carpe Diem Vascular meeting in Barcelona, Spain.
Z-Trak Plus Introduction System provides trackability and manoeuvrability for precise, controllable device orientation and deployment of the company's thoracic aneurysm stent graft
"...This monitor will be easy for my patients to use at home, and is expected to give early warning of the need for treatment, avoiding hospitalisation and deterioration in the patient's condition."
"The Valiant stent graft has quickly become the most widely used thoracic graft outside the United States..."
The results from three studies revealed that stenting is a safe and effective treatment option for treating SFA lesions
Presentation of results from 2 studies at this years annual meeting of the Vascular Society.
New UK subsidiary will begin sales of VNUS Closure products directly in the UK market 2007
The conference featured talks and demonstrations from the experts in the field from UK, Europe and USA
ESVS meeting, delegates were honoured to have a distinguished panel before them.
Study plans to enrol up to 50 patients with chronic deep venous insufficiency and venous ulcers (present for at least six months)
Venous Registry is to produce an annual publication similar to the vascular and adult cardiac surgical reports
Clot Lysis and Thrombolytic Therapy
Dangers of leaving the decision in primary care
Vascular News highlights the latest research developments for the treatment of varicose veins
Presentations made at the American Venous Forum
The results from the New ERA Study
Results presented by Dr Martin Schillinger
Presented at Charing Cross by Dr Ron Fairman
Optimal treatment strategy for patients with asymptomatic carotid disease
Vascular News spoke to Tom O'Donnell
The ESCHAR study results show that superficial venous surgery reduces venous ulcer recurrence.
Comparing the long term results of endovenous laser and endovenous RFA Therapy
A debate on the possible impact of the 48-hour EU directive