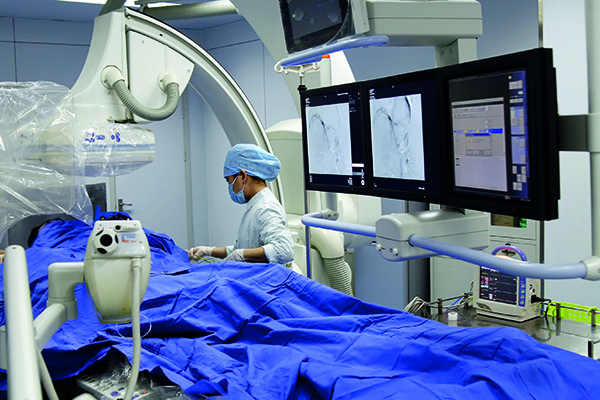
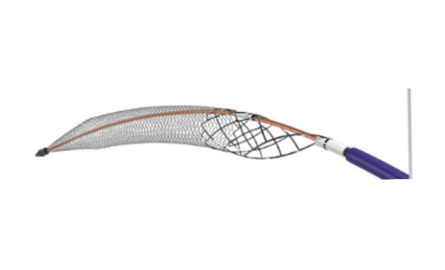
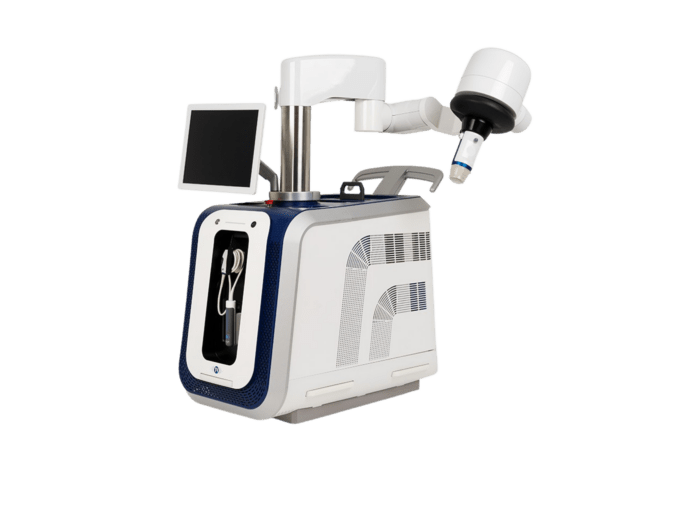
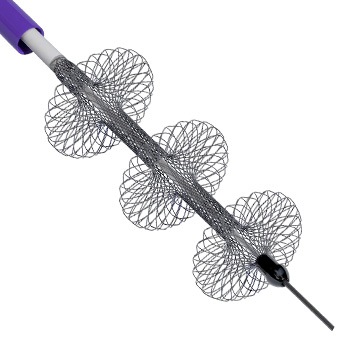
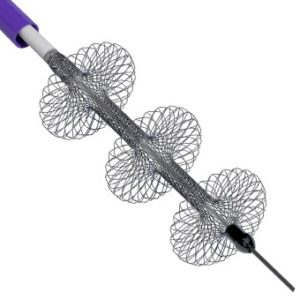
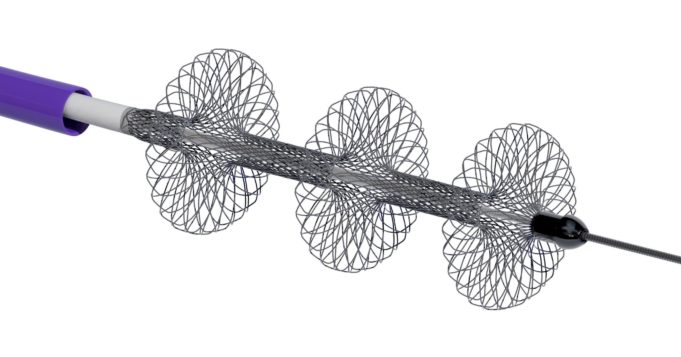
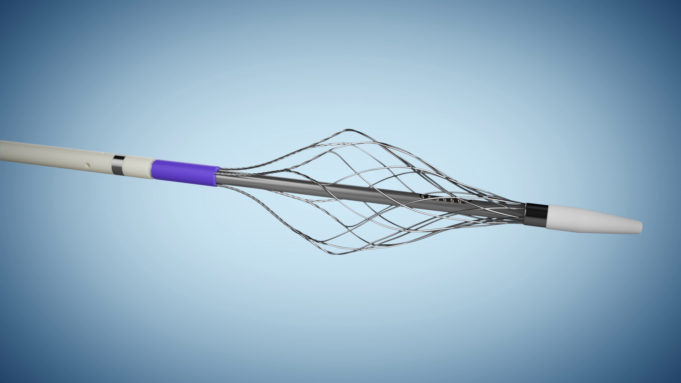
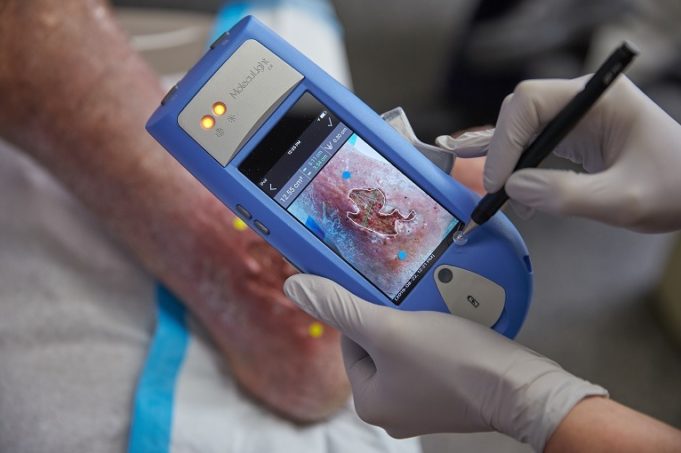
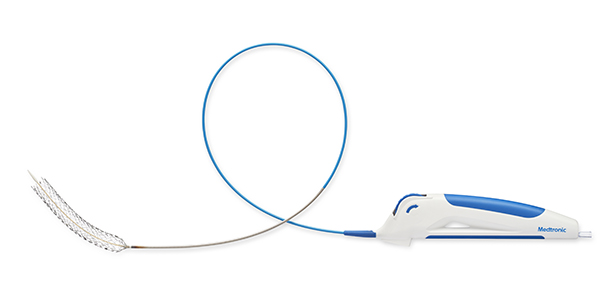
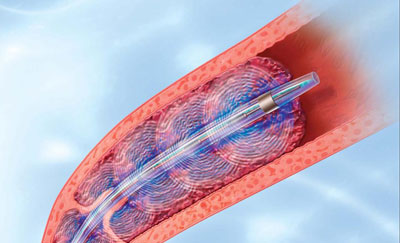
AngioDynamics recently announced the first patient enrolment in RECOVER-AV—a prospective, multicentre, multinational, single-arm study evaluating the AlphaVac multipurpose mechanical aspiration (MMA) F1885 system in the treatment of acute, intermediate-risk pulmonary embolism (PE).
The study follows the US Food and Drug...
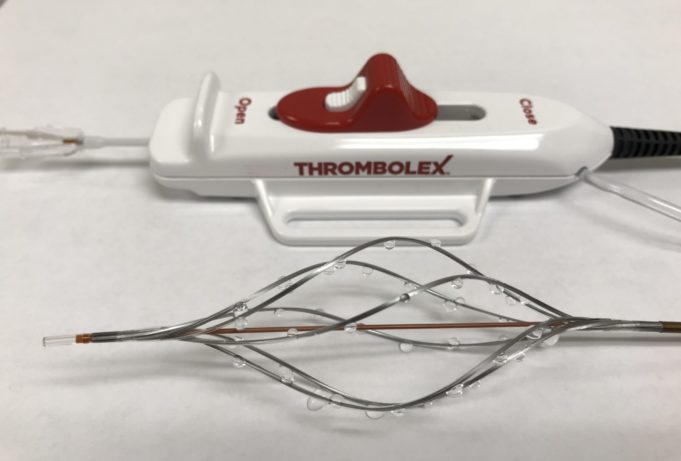
Thrombolex recently announced the publication of RESCUE-II study results in JACC: Advances.
The RESCUE-II study was a single-centre, prospective study evaluating the safety and feasibility of on-the-table (OTT) pharmacomechanical lysis (PML) without postprocedural infusion when treating patients with acute...
The Society for Cardiovascular Angiography & Interventions (SCAI) has released new, evidence-based clinical practice guidelines to support the treatment of chronic venous disease (CVD).
Published in JSCAI with an accompanying technical review, the document offers recommendations on a range of...
Basis Medical has announced the successful completion of its first-in-human clinical procedures using the Seclusion catheter to treat chronic venous insufficiency (CVI) caused by superficial vein reflux.
The cases were performed by Marcos Fletcher (Ciudad de Salud Hospital, Panama City,...
Embolization recently announced it has recevied 510(k) clearance from the US Food and Drug Administration (FDA) for its Nitinol Enhanced Device (NED)—a vascular embolisation device intended for arterial and venous embolisation in peripheral vasculature.
Using proprietary shape-memory biocompatible polymers, Embolization’s coil devices...
Endovascular Engineering (E2) has completed patient enrolment in the pivotal cohort of its ENGULF trial, involving the Hēlo pulmonary embolism (PE) thrombectomy system.
The investigational device exemption study was carried out at 19 interventional cardiology, radiology, and vascular surgery centres...
A recent research article has detailed an artificial intelligence (AI) model that enables physicians to predict which patients with superficial venous insufficiency have a higher risk of developing deep vein thrombosis (DVT) following endovenous thermal ablation (EVTA).
Published in...
New regulations have come into effect in the UK which place a greater emphasis on medical device manufacturers to monitor the safety and performance of their products.
The new Post-Market Surveillance (PMS) regulations came into effect from Monday (16 June)...
Inquis Medical has announced that its Aventus thrombectomy system has received 510(k) clearance from the US Food and Drug Administration (FDA) for an expanded indication to treat pulmonary embolism (PE).
The Aventus System is a next-generation mechanical thrombectomy platform developed...
Implantation of the VenoValve (enVVeno Medical) continues to promote stabilisation of symptoms in patients with deep venous reflux at two-year follow-up. This is the headline finding of data shared at the 2025 Vascular Annual Meeting (VAM; 4–7 June, New...
Makis Avgerinos (Athens, Greece), co-director of the venous programme for the European Vascular Course (EVC), speaks to Venous News at this year’s meeting (9–11 March, Maastricht, The Netherlands).
Avgerinos outlines some highlights the venous agenda at EVC 2025, including a...
"The path from initial trials to meaningful clinical data will not be easy,” writes Fedor Lurie (Toledo, USA) of new venous valve technologies.
There is an old saying that goes, “As surgeons, we change the anatomy to improve physiology”. Correction...
Envveno Medical has today announced that its manuscript titled, ‘Three-year outcomes of surgical implantation of a novel bioprosthetic valve for the treatment of deep venous reflux’, has been published in the in the peer-reviewed journal, Annals of Vascular Surgery.
The VenoValve was...
Imperative Care today announced the completion of patient enrolment in its SYMPHONY-PE study, a pivotal investigational device exemption (IDE) trial evaluating the safety and efficacy of the company’s Symphony thrombectomy system for the treatment of acute pulmonary embolism (PE).
Designed...
Inquis Medical recently announced results from its AVENTUS trial evaluating the safety and efficacy of the company’s Aventus thrombectomy system.
The results were presented by Jun Li (University Hospitals Harrington Heart & Vascular Institute, Cleveland, USA), the trial’s national co-principal...
VVT Medical recently announced the appointment of Antonios Gasparis to its medical advisory board.
A press release notes that Gasparis brings over two decades of expertise in vascular surgery, with a distinguished career dedicated to advancing the treatment of venous...
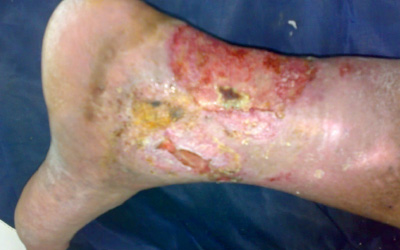
Twelve-month outcomes of the VenaSeal Spectrum venous leg ulcer (VLU) trial, assessing time to ulcer healing following treatment with the VenaSeal (Medtronic) closure system, have demonstrated an 81.3% ulcer healing rate at one year.
Manj Gohel (Cambridge, United Kingdom) presented...
In response to an audience poll during Thursday morning’s venous and lymphatic programme, 72% of respondents either disagreed or strongly disagreed with the statement ‘In my country, the leg ulcer pathway is working well’, highlighting the urgency of an...
A new subanalysis of the SAVVE (Surgical antireflux venous valve endoprosthesis) trial found that there was no difference in the level of improvement in clinical outcomes and quality-of-life measures between patients who have either primary or thrombotic deep venous...
Recently presented at the Society of Interventional Radiology (SIR) annual scientific meeting (29 March–2 April, Nashville, USA), data from the Jet Enhanced Thrombectomy intervention Hydrodynamic Thrombectomy System (JETi; Abbott) registry has demonstrated the device’s effectiveness having surpassed prespecified safety...
Charting the development of the Charing Cross (CX) venous and lymphatic programme from a sidebar event to becoming an “integral part” of the meeting’s agenda, executive board member Stephen Black (Guy’s and St Thomas’ NHS Foundation Trust and King’s...
Imperative Care today announced US Food and Drug Administration (FDA) 510(k) clearance of the 82cm version of its Symphony 16Fr catheter, the company’s latest innovation designed to elevate care for patients with venous thrombosis.
“Thrombectomy procedures have emerged as one...
Addressing the audience at the 28th European Vascular Course (EVC 2025; 9–11 March, Maastricht, The Netherlands), Ulka Sachdev-Ost (University of Pittsburgh, Pittsburgh, USA) set out a method to ensure scarce funding is directed to the most pressing venous disease...
SonoVascular has announced the successful completion of an initial set of eight deep vein thrombosis (DVT) cases using its SonoThrombectomy system as part of a first-in-human (FIH) study of the device.
All cases achieved a complete Marder score reduction as...
Kathleen Ozsvath (New York, USA), past president of the Eastern Vascular Society (EVS), is an esteemed vascular surgeon with expertise in the treatment of superficial and deep venous disease, including long-term sequelae of chronic venous disease. Mapping her career...
Stereotaxis has announced a US Food and Drug Administration (FDA) regulatory submission for the first robotically navigated catheter designed to expand usage of robotic magnetic navigation into the broader endovascular field.
Emagin 5F is the first in a family of...
Basis Medical recently announced that its Seclusion catheter for superficial vein reflux has received US Food and Drug Administration (FDA) 510(k) clearance.
The company notes that the Seclusion catheter is an endovenous, chemical ablation device designed to provide a safer,...
The need for a revolutionary second generation of venous stents are among the gaps and unmet needs currently at play in deep venous surgery, the 2025 American Venous Forum (AVF; 16–19 February, Atlanta, USA) heard.
Erin Murphy (Atrium Health’s Sanger...
A multisite hospital system analysis of the safety and procedural learning curve behind the performance of percutaneous mechanical thrombectomy for pulmonary embolism (PE) shows significant reductions in both fluoroscopy and procedure times alongside contrast volume over time.
The data, the...
With the aims of predicting and comparing venous stent outcomes, aiding in communication with patients, and enhancing therapeutic decision-making, researchers have proposed an anatomical classification system for patients with chronic venous obstruction (CVO) of the iliofemoral tract undergoing interventional...
One-year follow-up data on 75 subjects from the VenoValve (Envveno Medical) US pivotal trial were presented by principal investigator Cassius Iyad Ochoa Chaar (Yale School of Medicine, New Haven, USA) at the 2025 American Venous Forum (AVF; 16–19 February, Atlanta,...
Stryker announced today that it has completed the acquisition of Inari Medical. A press release notes that the addition of Inari brings an established peripheral vascular position to Stryker in the fast-growing venous thromboembolism (VTE) segment.
“The acquisition of Inari...
Endovascular Engineering (E2) has secured US$42 million in an oversubscribed Series B financing to advance its next-generation clot removal technology platform for venous thromboembolism (VTE), a press release reports.
E2 shares that the round was co-led by 415 Capital...
Akura Medical has announced today the first patient enrolment in the QUADRA-PE study evaluating the Katana thrombectomy system in patients with acute pulmonary embolism (PE). The initial procedure was successfully performed by Samuel Horr, director of cardiovascular research at TriStar...
Penumbra recently announced the launch of its Element vascular access system. This is the first laser-cut hypotube sheath designed for venous thromboembolism (VTE), the company shares in a press release.
Penumbra notes that Element is compatible with the Lightning Flash...
A systematic review and meta-analysis of over 1,500 venous stenting procedures—said to be the first study on this topic to date—has highlighted an 18% drop in primary patency rates after one-year follow-up with declining rates beyond 12 months. As...
A large nationwide survey conducted by the Japanese Regulatory Committee for Endovascular Treatment of Varicose Veins has demonstrated that cyanoacrylate closure (CAC) for varicose veins is safe with low rates of serious adverse events, such as venous thromboembolism.
CAC...
The US Food and Drug Administration (FDA) has issued a statement outlining measures to enhance protections against medical device shortages.
According to Michelle Tarver, director of the FDA’s Center for Devices and Radiological Health (CDRH), supply chain disruptions—often caused...
Piccolo Medical has announced an exclusive distribution agreement with Spectrum Vascular. Spectrum Vascular offers a portfolio of venous catheters that leverage the Spectrum antimicrobial and BioFlo thrombus reduction technologies.
Under the partnership, Spectrum Vascular will serve as the sole distributor...
Researchers in Canada have developed machine learning (ML) models that they claim, “can accurately predict one-year IVC filter complications, performing better than logistic regression”.
Writing in the Journal of Vascular Surgery: Venous and Lymphatic Disorders (JVS-VL), Ben Li (University...
Argon Medical announces the first patient enrolment in the CLEAN-PE study. The prospective, multicentre CLEAN-PE study aims to evaluate the safety and efficacy of the Cleaner Pro thrombectomy system for removing blood clot from the lungs in patients diagnosed...
The US Food and Drug Administration (FDA) has issued draft guidance that includes recommendations to support development and marketing of safe and effective artificial intelligence (AI)-enabled devices.
The guidance, if finalised, would be the first guidance to provide comprehensive...
Stryker has announced a definitive agreement to acquire all of the issued and outstanding shares of common stock of Inari Medical for US$80 per share in cash, representing a total fully diluted equity value of approximately US$4.9 billion.
A Stryker...
Data from a new study have found that venous leak embolization yields additional clinical improvement and treatment potential in patients with vasculogenic erectile dysfunction (ED) resistant to phosphodiesterase-5-inhibitors (PDE5i) due to mixed arterio-venous disease. These findings were recently published...
Before the STEVECO trial, there were no randomized controlled trial data in the chronic disease space; in its wake, has come a more robust landscape to establish an evidence base: During the 2024 Charing Cross (CX) International Symposium (April...
Flow Medical today announced the closing of an oversubscribed US$5 million seed funding round, which includes US$2 million previously secured from friends and family. A press release notes that this investment positions Flow Medical to accelerate the development and...
At the recent VEITHsymposium (19–23 November, New York, USA), Marc Passman (University of Alabama at Birmingham, Birmingham, USA) reflected on the achievements of the Society for Vascular Surgery (SVS) Vascular Quality Initiative (VQI) venous arm, in collaboration with the...
Inari Medical has announced that it received national reimbursement approval from the Japanese Ministry of Health, Labor and Welfare (MHLW) for its ClotTriever thrombectomy system for deep vein thrombosis.
A recent press release states that, due to ClotTriever’s mechanism...
Envveno Medical has announced that it will present one-year data on all patients from the VenoValve US pivotal trial today at the VEITHsymposium (19–23 November, New York, USA). The definitive one-year data support the application submitted earlier this week...
enVVeno Medical today announced it has submitted an application with the U.S. Food and Drug Administration (FDA) seeking approval to market the VenoValve—a surgical venous valve implant for chronic venous insufficiency—in the USA. Definitive one year data from the...
Ten-year data from the randomised HELP trial show that, while both endothermal ablation and conventional surgery are effective treatments for great saphenous varicose veins at 10 years, the former was associated with superior clinical and quality-of-life (QoL) outcomes. These...
Penumbra has announced new data that demonstrate patients with intermediate-risk pulmonary embolism (PE) treated with Penumbra's computer-assisted vacuum thrombectomy (CAVT) technology have a shorter length of hospital stay, shorter post-procedure length of stay and fewer complications when compared to...
Inquis Medical has announced the successful closure of its US$40 million series B financing round. This round was led by Marshall Wace, a globally recognised investment firm, with participation from existing investors, including ShangBay Capital, Yu Star, EnPointe Ventures,...
The US Food and Drug Administration (FDA) has approved Akura Medical’s investigational device exemption (IDE) application to initiate the QUADRA-PE study evaluating the Katana thrombectomy system in patients with acute pulmonary embolism (PE).
The co-principal investigators of the pivotal study...
Findings from the first international randomised controlled trial (RCT) to compare patient outcomes following treatment with large-bore mechanical thrombectomy (LBMT) versus catheter-directed thrombolysis (CDT) for intermediate-risk pulmonary embolism (PE) found that LBMT is superior with respect to the hierarchically-tested...
Cook Medical’s Zilver Vena venous self-expanding stent has shown high rates of patency sustained for three years, according to recently published data in the Journal of Vascular and Interventional Radiology (JVIR). The patency results extended across all patient subgroups, and patients experienced improvements in...
Thrombolex has announced the enrolment of the first two patients in the RAPID-PE study using the Bashir endovascular catheter for the treatment of acute pulmonary embolism (PE). The patients were enrolled by Ayman Iskander (St Joseph’s Health Hospital, Syracuse,...
“One of the great and simultaneously worrisome things about venous disease is that we’re still in the first generation of our understanding of pathophysiology and therapies. It’s really exciting in the sense that there’s so much more to go...
Results from a subset analysis of the ABRE study have shown that patients in three out of the six World Health Organisation (WHO) body mass index (BMI) groups—‘pre-obesity’, ‘obesity class I’ and ‘obesity class II’—trended towards higher 36-month patency...
Sonablate has announced the first patient enrolled in the HIFIVE clinical trial in the USA under the guidance of principal investigator, Naiem Nassiri (The Vascular Care Group, Darien, USA).
As part of this new trial, effective HIFU delivery to...
This advertorial is sponsored by Inari Medical.
Evaluating the current standard of care for the management of acute pulmonary embolism (PE) and deep vein thrombosis (DVT), Michael Kostrzewa (Cantonal Hospital Baden, Baden, Switzerland) and Rashid Akhtar (Barts Health NHS Trust,...
In recognition of World Thrombosis Day, Inari Medical recently announced collaborative partnerships with the American Venous Forum (AVF) and the National Blood Clot Alliance (NBCA) on the DEFIANCE trial.
DEFIANCE is a prospective, multinational, randomised controlled trial (RCT) that...
A new paper that drills into the impact of a novel bioprosthetic venous valve replacement by CEAP (Clinical, Etiological, Anatomical and Pathophysiological) classification shows sustained clinical improvement regardless of grading, with patients classed as C4b exhibiting greater resolution of...
Published in the Journal of Thrombosis and Thrombolysis, new research with a follow-up of 27 years has found an association between high levels of physical activity (PA) and an elevated risk of venous thromboembolism (VTE).
The research, led by Per...
Tactile Medical has announced that Nimbl, its next-generation pneumatic compression platform, is now commercially available throughout the USA for the treatment of upper-extremity lymphoedema.
Nimbl, which the company notes is significantly smaller and lighter than previous device iterations, is...
InterVene recently announced the closing of its US$13 million Series A financing round. The financing was co-led by new investor Treo Ventures and existing investor RiverVest Venture Partners. In conjunction with this financing, Brad Vale, Treo Ventures founding general...
Surmodics has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance for its Pounce XL thrombectomy system.
The Pounce XL thrombectomy system is indicated for the non-surgical removal of thrombi and emboli from the peripheral arterial...
A new deep vein thrombosis (DVT) diagnostic pathway incorporating non-expert artificial intelligence (AI)-guided compression ultrasound could reduce workload and costs for healthcare systems while providing a quicker diagnosis and improving patient care.
Efthymios (Makis) Avgerinos (Athens, Greece) shared this...
B Braun recently announced that the US Food and Drug Administration (FDA) has granted 510(k) clearance for the Introcan Safety 2 deep access intravenous (IV) catheter, the newest addition to the Introcan Safety 2 IV catheter portfolio.
The Introcan Safety...
A pilot study in Canadian capital Ottawa aimed at uncovering the extent of undiagnosed vascular disease among the city’s homeless population detected a subset who may be in need of treatment for venous disease.
The work was carried out by...
Argon Medical has today announced the launch of the Cleaner Vac thrombectomy system for the removal of blood clot from the peripheral venous vasculature.
The Cleaner Vac thrombectomy system is a disposable, large-bore aspiration system designed to quickly and effectively...
Penumbra today announced it has secured CE mark in Europe for two computer-assisted vacuum thrombectomy (CAVT) technologies—Lightning Flash 2.0 and Lightning Bolt 7.
“Based on outcomes from our clinical trials and from physicians using our devices globally, our CAVT technologies...
A recent analysis of deep venous stent placement has determined that the procedure is safe with low rates of major complications. Published in the journal CardioVascular and Interventional Radiology, the research team, led by Doireann P Joyce (Galway University...
A recent study published in the Journal of Thrombosis and Thrombolysis has revealed a significant decrease in the incidence of mortality and major bleeding in patients with COVID-19-associated venous thromboembolism (VTE) in the period between 2021–2022 when compared with 2020....
In this short video, Michael Kostrzewa (Baden, Switzerland) and Rashid Akhtar (London, UK) discuss the latest approaches to the treatment of acute pulmonary embolism (PE) and deep vein thrombosis (DVT) using mechanical thrombectomy.
They examine which patients are eligible...
Koya Medical recently announced that full results from the TEAYS clinical study have been published in the Journal of Vascular Surgery (JVS).
The data highlight superior efficacy and improved quality of life for patients suffering from lower extremity lymphoedema...
VVT Medical recently announced that the American Venous Forum (AVF) has provided an official opinion letter regarding CPT coding guidance for the ScleroSafe percutaneous endovenous ablation procedure. This procedure, which combines mechanical collapse and chemical ablation to treat incompetent...
The pilot phase of a new European Venous Registry (EVeR)—designed to illuminate the real-world outcomes of deep venous interventions over a 10-year time horizon—is set to launch this month (September 2024).
Developed and hosted by the European Society for Vascular...
Jupiter Endovascular has announced that the US Food and Drug Administration (FDA) has approved its Investigational Device Exemption (IDE) application for the SPIRARE II US pivotal study. The pivotal trial will study the Vertex pulmonary embolectomy system, which incorporates Jupiter’s Endoportal Control platform technology into endovascular...
Obvius Robotics has announced that the US Food and Drug Administration (FDA) has granted Breakthrough Device designation for its CERTA access system for central venous catheterisation (CVC).
Breakthrough Device designation expedites the review of innovative technologies that provide for more...
SonoVascular has announced the successful initiation of its first-in-human study (FIH) of the company's SonoThrombectomy system, an ultrasound-facilitated, thrombolytic-enhanced platform that utilises multiple mechanisms of action to treat patients with venous thromboembolism (VTE). The catheter-based system combines microbubble-mediated cavitation...
The US Food and Drug Administration (FDA) has issued a Class I recall—the most serious type—for Inari Medical's ClotTriever XL, 30mm device due to reports of patient injury, and death from device entrapment and pulmonary emboli (PE).
The device remains...
Endovascular Engineering (E2), which specialises in the advancement of clot removal technologies for venous thromboembolism (VTE), has named Dan Rose as chief executive officer (CEO). Former CEO and founding team member Mike Rosenthal will continue as chief operating officer...
The Eastern Vascular Society (EVS) has incorporated a day of service into its 2024 annual meeting, being held in Charleston, South Carolina (Sept. 19–22).
Leading vascular surgeons, alongside nurse practitioners and advanced practice partners, will educate local residents about their...
The diagnosis and management of non-thrombotic iliac vein lesions (NIVL) is the focus of a newly published set of consensus statements from the Vascular Interventional Advances (VIVA) Foundation, the American Venous Forum (AVF), and the American Vein and Lymphatic...
VVT Medical has announced a milestone in its production and cost reduction initiatives.
A press release reports that, in July 2024, the company inaugurated a new catheter production line in Mauritius.
VVT Medical details that this production site offers ultramodern manufacturing...
Thrombolex and Aidoc have announced a strategic partnership aimed at revolutionising the treatment of acute pulmonary embolism (PE), a press release reports.
This strategic collaboration, the release details, will leverage Aidoc’s artificial intelligence (AI) technology to accelerate patient identification and...
At the recent Endo Vascular Access (EVA) meeting (14–15 June, Patras, Greece), Ziv Haskal (University of Virginia School of Medicine, Charlottesville, USA) spoke to Timothy Clark (Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA) about what...
Theraclion, a French MedTech company developing a robotic platform for non-invasive high-intensity focused ultrasound (HIFU) therapy, announces today that treatments in the US Food and Drug Administration (FDA) pivotal study for Sonovein, an extracorporeal medical device made to treat varicose veins...
A recent study published in the Journal of Vascular Surgery: Venous and Lymphatic Disorders looked at varicose vein surgery after acute isolated superficial vein thrombosis (SVT) in daily practice, determining that the management of SVT should be guided by...
In this issue:
In Profile: Gloria Salazar (Chapel Hill, USA)
Gerry O'Sullivan (Galway, Ireland), Steve Black (London, UK), and Erin Murphy (Charlotte, USA) discuss stenting NIVL patients
Conference round-up: Highlights from IVC 2024
Peter Pappas (Chester, USA) gives 2024 Venous...
In this issue:
In Profile: Gloria Salazar (Chapel Hill, USA)
Gerry O'Sullivan (Galway, Ireland), Steve Black (London, UK), and Erin Murphy (Charlotte, USA) discuss stenting NIVL patients
Conference round-up: Highlights from IVC 2024
Peter Pappas (Chester, USA) gives 2024 Venous...
Deep vein thrombosis (DVT) is a significant health risk. In about half of patients, the blood clot breaks away from the vein wall and travels to the lungs, where it can cause a pulmonary embolism. Approximately 25% of people...
This advertorial is sponsored by Optimed.
Before the STEVECO trial, there were no randomized controlled trial data in the chronic disease space; in its wake, has come a more robust landscape to establish an evidence base: During the 2024 Charing Cross...
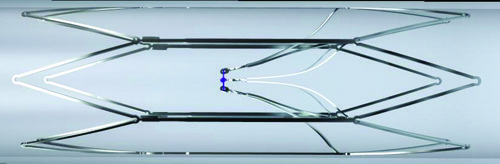
Royal Philips today announced the first implant of the Duo venous stent system, an implantable medical device indicated to treat symptomatic venous outflow obstruction in patients with chronic venous insufficiency (CVI), following premarket approval (PMA) from the US Food...
Should non-thrombotic iliac vein lesion (NIVL) patients be given venous stents? That was the question raised to Gerry O’Sullivan (Galway, Ireland), a consultant interventional radiologist at the University Hospital Galway, and Stephen Black (London, UK) a consultant vascular surgeon...
“There are a lot of patients walking out there who are not being treated. So you can only imagine the impact that we can have as specialists to be able to improve those patients’ quality of life,” Gloria Salazar...
In conversation with Venous News at Paris Vascular Insights (PVI) 2023 (8–10 November, Paris, France), Michael Lichtenberg (Arnsberg, Germany) spoke at length on the state of play in the venous field.
The chief medical officer and director of the Angiology Department and...
At the Leipzig Interventional Course (LINC) 2024 (May 28–31, Leipzig, Germany), Michael Lichtenberg (Arnsberg Vascular Clinic, Arnsberg, Germany) shared for the first time a 24-month update from the P-MAX study of the Aspirex (BD) endovascular thrombectomy system in the...
With seven compassionate-use cases under its belt and commercialization in view, VeinWay—developer of the Traversa, a tool for crossing tough venous occlusions—is at a crucial point in its evolution. That is according to chief executive officer (CEO) and co-founder...
The 2024 Venous Symposium (May 8–11, New York City, USA) was highlighted by keynote speaker Peter Pappas (Chester, USA), regional medical director and program director of the Venous and Lymphatic Medicine Fellowship at the Center for Vein Restoration. Pappas...
Experience wall-to-wall thrombectomy in action with Manuela Konert from Leipzig, Germany! In this brief video, Konert walks through a deep vein thrombosis (DVT) case step by step using the ClotTriever system, providing valuable clinical insights along the way. Highlighting...
Results from Acute Pulmonary Embolism Extraction Trial with the AlphaVac System (APEX-AX) finds significant improvement in right ventricle function and minimal major adverse events.
New data from APEX-AV demonstrated that catheter-directed mechanical thrombectomy is safe and effective in patients with...
New data from the REAL-PE analysis investigated catheter-based pulmonary embolism (PE) treatment, showing women and Black people were less frequently treated with minimally invasive therapy compared to men or non-Black patients. The late-breaking results were presented today at the...
Late-breaking data from the ENGULF trial showed that a novel dual-action thrombectomy device was effective and safe in treating acute pulmonary embolism (PE). The safety and effectiveness results were presented today as late-breaking science at the Society for Cardiovascular...
On the final day of the 2024 International Vein Congress (April 18–20) in Miami, Florida, Joseph P. Hart, MD, associate professor of surgery and radiology at Medical College of Wisconsin in Milwaukee, Wisconsin, Andrea P. Munive-Gnecco, MD, from Bogotá,...
Expanse Ice recently announced that its Ice aspiration system has received 510(k) clearance from the US Food and Drug Administration (FDA).
A press release notes that the Ice system is specifically designed to address the complex challenges associated with peripheral...
VVT Medical has announced the completion of GMP certification and registration of Novel Non-Thermal, Anesthesia- free treatment for Varicose veins. This milestone approval adds to the recent FDA 510(k) clearance and existing CE Mark, reinforcing ScleroSafe's position as a...
The importance of activating the calf muscle pump in the treatment of lower extremity lymphedema came to the fore during the 2024 International Vein Congress (IVC) in Miami, Florida (April 18–20).
“There are a whole host of non-proffered approaches you...
“The surgical challenges of repairing deep venous reflux have been present for more than a generation. It is worth reflecting on the fact that this new technology has made the majority of patients vastly better,” were the words of...
Patients undergoing cyanoacrylate glue closure for superficial venous disease reported higher periprocedural satisfaction than those who were treated via surgical stripping, data from a pair of randomised controlled trials (RCTs) assessing the VenaSeal system (Medtronic) reveal.
The modality also showed...
Twelve-month results from the SYNCHRONOUS trial, which is evaluating the role of prophylactic anterior accessory saphenous vein (AASV) ablation for the prevention of recurrent varicose veins, show superiority among the group of patients whose AASV was ablated at the...
Penumbra has announced the US Food and Drug Administration (FDA) clearance and launch of Lightning Flash 2, the next generation computer assisted vacuum thrombectomy (CAVT) system to remove venous thrombus and treat pulmonary embolism (PE), a press release states.
Lightning...
During the 2024 International Venous Conference (April 18-20) in Miami, Florida, Lowell Kabnick, MD (Lake Worth Beach, Florida), gave s presentation on the newest venous devices currently in development.
“We’re going to look into the future a little bit,” Kabnick...
Patrick Muck (Cinncinatti, Ohio) provided a state of the venous stent landscape during the 2024 International Vein Congress (IVC; April 18–20) in Miami.
The TriHealth vascular surgery program director ran through the key differences between the venous-specific stents currently available...
SCVS 2024 saw Zachary AbuRahma, DO, an assistant professor of vascular surgery at West Virginia University and the Charleston Area Medical Center in Charleston, West Virginia, presented data from a single-center experience focused on the use of the FlowTriever...
Erin Murphy (Atrium Health’s Sanger Heart and Vascular Institute, Charlotte, USA), one of the CX Symposium cochairs and a CX Executive Board member, along with fellow CX Executive Board members Stephen Black (Guy’s and St Thomas’ Hospital; King’s College...
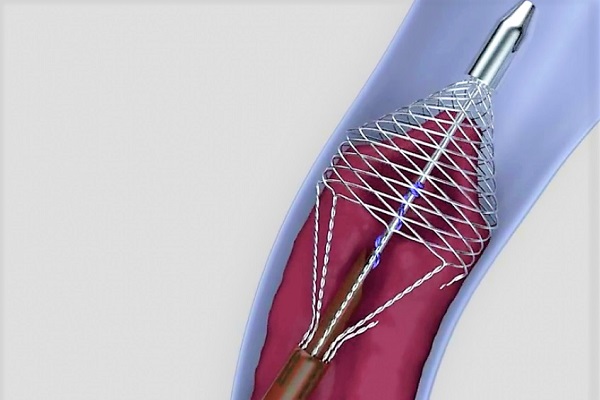
AngioDynamics has announced that the United States Food and Drug Administration (FDA) has cleared their AlphaVac F1885 system for the treatment of pulmonary embolism (PE).
According to the press release, the AlphaVac F1885 System is a non-surgical alternative for the...
Evaluation of the safety and performance of a novel pharmaco-mechanical procedure for acute deep vein thrombosis (DVT) in two pigs demonstrated a significant reduction in thrombus burden and vessel wall preservation, data from in vivo testing reveals.
The...
During a presentation at the 2024 European Vascular Course (EVC; March 3–5) in Maastricht, The Netherlands, Anna Pouncey, a clinical research fellow for vascular surgery at Imperial College London in London, England, presented findings from a study on the...
In a recent interview, Marie Josee van Rijn from Rotterdam, the Netherlands and Emma Wilton from Oxford, UK, provided insight into the transformative power of interventional approaches in the treatment of DVT. They also set the stage for the...
Medical imaging AI company Avicenna.AI has announced 510(k) clearance from the US Food and Drug Administration (FDA) for its CINA-iPE artificial intelligence (AI)-powered tool that detects incidental pulmonary embolism (PE) during routine computed tomography (CT) scans.
The CINA-iPE algorithm identifies lung blood...
Manj Gohel (Cambridge, UK), member of the Venous Executive Board, parachutes straight into the CX Venous and Lymphatics Controversies Programme to highlight the VenaSeal SPECTRUM programme, SAVVE trial and SYNCHRONOUS trial, which will all present results for the very first...
Twelve-month outcomes from the Pounce venous thrombectomy system (Vetex Medical) first-in-human study evaluating its use in the treatment of acute iliofemoral deep vein thrombosis (DVT) recently published in the Journal of Vascular Surgery: Venous and Lymphatic Disorders (JVS-VL) demonstrated...
During the 2024 American Venous Forum (AVF) annual meeting in Tampa, Florida (March 3–6), Kurt S. Schultz, MD, a general surgery resident at the Yale University School of Medicine in New Haven, Connecticut, presented data from a study regarding...
Rates of post-thrombotic syndrome (PTS) among deep vein thrombosis (DVT) patients treated with the ClotTriever thrombectomy system (Inari Medical) who are logged in the real-world CLOUT registry demonstrated "significant and sustained improvement" out to 24 months, according to interim...
At the 2024 American Venous Forum (AVF) in Tampa, Florida (March 3–6), Glenn Jacobowitz, MD, professor of vascular and endovascular surgery at NYU Langone Health in New York City and 2023-24 AVF president, tackled the future direction of the...
A new study presented during the 2024 American Venous Forum (AVF) in Tampa, Florida (March 3–6), that compared a novel non-pneumatic compression device with an advanced pneumatic compression modality in ther treatment of lower extremity lymphedema demonstrated “statistically significant...
An abstract presented at the 2024 American Venous Forum (AVF) annual meeting (March 3–6) revealed that, in the treatment of patients with pulmonary embolism (PE), the implementation of artificial intelligence (AI) software has been linked to a shorter time...
A nationwide US study, recently published in the Journal of Vascular Surgery: Venous and Lymphatic Disorders (JVS-VL), showed that Black patients had the highest inferior vena cava (IVC) filter placement rate per 100,000 persons compared with white and Latino...
A large international Delphi consensus on the management of lower extremity venous outflow obstruction has been published, identifying “clear areas of agreement and disagreement” between experts to support future research and writing of guidelines in this field. The consensus...
“As I learned more about venous pathologies, it became abundantly clear that patients with venous disease were a largely neglected population in need of strong, passionate advocates,” Manj Gohel (Cambridge and London, UK) tells Venous News, recalling his decision...
The quest for more data—specifically multicenter, independently adjudicated and, where possible, randomized—in the field of venous disease continues, with various trials currently underway set to provide some eagerly anticipated evidence. The path to best practice is long and winding,...
“Expect—and prepare for—the unexpected,” Karen Gibson, Sergeant at Arms at the United States Senate, told the UIP 2023 World Congress (Sept. 17–21) during a keynote address entitled ‘Leadership in crisis’. In this interview with Venous News, Gibson summarizes some...
Mona Gupta (Chicago, USA) writes about the importance of acknowledging the “complex relationship” between the deep and superficial venous systems.
The complex relationship between the deep and superficial venous systems is an important one to recognize and understand in order...
Mechanical thrombectomy using the ClotTriever device (Inari Medical) for iliofemoral deep vein thrombosis (DVT) was found to be “associated with significantly” lower Villalta scores and a lower incidence of post-thrombotic syndrome (PTS) out to 12 months compared with treatment using...
The pitfalls of large administrative databases came to the fore during the presentation of a propensity-match scoring analysis demonstrating that percutaneous mechanical thrombectomy (MT) was superior to catheter-directed thrombolysis (CDT) in patients diagnosed with acute pulmonary embolism (PE). Junji...
In a retrospective review of over 600 patients who underwent iliac vein stent placement, the development of back pain was found to be unrelated to stent type, diameter, length or covered vein territory.
Chloe Snow (Greenbelt, USA) and colleagues share...
This advertorial, sponsored by Optimed, is only available in selected countries and geographies.
One-year results from the STEVECO randomized controlled trial (RCT) were recently published, providing some much-needed data on the role of venous stenting for patients with chronic...
In this issue:
In the making: An in-depth look at two trials that are set to provide some much-needed data for the treatment of venous disease
Conference round-up: Highlights from The VEINS and VIVA 2023
In profile: Manj Gohel (Cambridge and London, UK)
Mona...
In this issue:
In the making: An in-depth look at two trials that are set to provide some much-needed data for the treatment of venous disease
Conference round-up: Highlights from The VEINS and VIVA 2023
In profile: Manj Gohel...
NOTE: This video is ONLY available to watch in selected countries and geographies
David Dexter, (Sentara Vascular Specialists, Norfolk, USA, National Co-PI of the CLOUT registry) speaks on recently presented one-year outcomes from the 500 patient CLOUT registry (NCT03575364)....
Innova Vascular has announced successful early commercial use of the company's Laguna thrombectomy system. Physicians at UCLA Medical Center in Los Angeles, USA, and Providence St Joseph in Orange, USA, who were the first to use this system in...
A new scientific statement from the American Heart Association (AHA) emphasises the need to boost patient and physician awareness of cerebral venous thrombosis (CVT), with a view to improving recognition of this condition and initiation of prompt medical treatment....
VVT Medical recently announced the signing of a strategic distribution agreement with Methapharm, a specialty pharmaceutical company.
According to a press release, this collaboration marks a pivotal moment in the realm of varicose vein treatment, leveraging the strengths of both...
Stephen Black speaks to Venous News about his career to date. Despite having, in his own words, done “terribly” in an interview for a vascular senior house officer role at St Mary’s Hospital in London, England, Black is now...
Endovascular Engineering (E2) has announced US Food and Drug Administration (FDA) investigational device exemption (IDE) approval for its ENGULF US pivotal trial.
The study will evaluate the safety and effectiveness of the Hēlo thrombectomy system in treating pulmonary embolism (PE)....
Proceedings from an expert consensus roundtable that discussed the benefits of intravascular ultrasound (IVUS) in lower extremity revascularization procedures were released today in the Journal of the Society for Cardiovascular Angiography & Interventions (JSCAI), Journal of Vascular and Interventional Radiology (JVIR), and Journal...
Which stories captured the attention of the venous community this year? Read our summary of the trending articles from across the Venous News network throughout 2023.
What were your highlights? Leave a reply at the foot of the page with...
Start with a duplex scan, continue with axial imaging, and then assess with venography and intravascular ultrasound (IVUS). This was the advice of Efthymios (Makis) Avgerinos, MD, speaking on the standard of care that is multimodal imaging in assessing...
Our biases permeate the fabric of our very being, as they weave their way into our training and practice. It is clear that complex aortic work is definitely in vogue and 'in', and, well... treating veins gets a bad...
Patient enrollment is now complete in the APEX-AV trial evaluating the safety and efficacy of the AlphaVac F1885 (AngioDynamics) multipurpose mechanical aspiration system for the treatment of acute intermediate-risk pulmonary embolism (PE).
APEX-AV is a single-arm investigational device exemption (IDE)...
A new intravenous anaesthesia delivery technique used during endothermal ablation for varicose veins demonstrated comparatively low pain scores according to first-in-human (FIH) data recently presented at the 2023 VEITHsymposium (14–18 November, New York, USA).
The investigational Solutio catheter (MedVasc), which...
Patients undergoing computer-assisted vacuum thrombectomy with the Indigo Aspiration System (Penumbra) for pulmonary embolism (PE) showed 2.7% rates of both major adverse events (MAEs) and major bleeding at 48 hours post-procedure alongside “a significant reduction” in right ventricle/left ventricle...
Forecasting their Charing Cross (CX) 2024 venous programme highlights, executive board members Manj Gohel (London, United Kingdom), Erin Murphy (Charlotte, USA) and Stephen Black (London, United Kingdom) discuss the “exciting” controversies and debates, first data releases and a “much...
The Duo venous stent system (Vesper Medical/Philips) showed a 98.7% freedom from major adverse events (MAEs) at 30 days and 90.2% primary patency at 12 months, results from the VIVID trial have revealed.
The data—which covered 162 subjects with nonthrombotic,...
Results from a first-in-human, prospective, single-arm study of the Akura Medical thrombectomy system (Akura Medical) for pulmonary embolism (PE) were revealed this week at The VEINS 2023 (28–30 October, Las Vegas, USA).
Presenter Jay Mathews (Manatee Memorial Hospital, Bradenton, USA)...
The open-label phase of the DEXTERITY-AFP trial investigating the Bullfrog microinfusion device—which involves the perivenous injection of the anti-inflammatory drug dexamethasone to improve patency and post-thrombotic syndrome (PTS) six months after thrombus removal in symptomatic deep vein thrombosis (DVT)...
David Dexter (Sentara Vascular Specialists, Norfolk, USA) shared one-year data from the CLOUT registry this week at The VEINS 2023 (28–30 October, Las Vegas, USA).
Long-term, prospective outcome data after mechanical thrombectomy for deep vein thrombosis (DVT) are lacking. The...
Vein360 has announced it received US Food and Drug Administration (FDA) 510(k) clearance for reprocessing the Philips Visions PV 0.035 digital intravascular ultrasound (IVUS) catheter in June of 2023 and the Visions PV 0.018 digital IVUS catheter in August...
In this issue:
The difficulties of moving from page to practice: New guidelines and the challenges of implementation
September conference round-up: Highlights from the International Union of Phlebology (UIP) world congress and the European Society for Vascular Surgery (ESVS) annual meeting
In...
In this issue:
The difficulties of moving from page to practice: New guidelines and the challenges of implementation
September conference round-up: Highlights from the International Union of Phlebology (UIP) world congress and the European Society for Vascular Surgery (ESVS) annual...
Data from the REAL-PE study were presented this week at TCT 2023 (23–26 October, San Francisco, USA) demonstrating that patients treated for pulmonary embolism (PE) with the Ekos endovascular system (Boston Scientific) had lower rates of adverse events, including...
This advertorial, sponsored by BD, is intended only for readers outside the USA.
“A dedicated, holistic venous approach is mandatory—including deep, superficial and pelvic venous systems,” says Houman Jalaie (University Hospital RWTH Aachen, Aachen, Germany), outlining what he believes was...
Thrombolex has announced never-before-reported major reductions in obstruction in all of the segmental pulmonary arteries (PA), based on independent core lab data analysis of 107 patients from 18 sites in the USA, with acute intermediate-risk pulmonary embolism (PE), using...
Roger Malcolm Greenhalgh, the surgeon internationally renowned for his unparalleled contribution to vascular education, training and research, died peacefully on 6th October, aged 82. At the time of his death, he was emeritus Professor of Surgery at Imperial College...
Research in the advancement of artificial intelligence (AI)-driven pulmonary embolism (PE) care was unveiled recently during the 9th Annual Pulmonary Embolism Symposium (Sept. 21–23) in Austin, Texas. A press release reports that research from three institutions utilizing Aidoc's PE...
“Occasionally, taking a step in a different direction can pave the way for substantial strides forward,” Erin Murphy, MD, tells Venous News, recalling her discovery of the venous world and a defining moment in her career. In this interview,...
Viz.ai has announced new clinical data supporting advancements in pulmonary embolism (PE) detection. Two studies have demonstrated the real-world clinical efficacy of Viz.ai's PE module to quickly and accurately identify PE and associated right heart strain, accelerate care coordination,...
The pursuit of a cure for deep venous valvular reflux—long considered to be the “holy grail” of deep venous disease—is underway, with new technologies set to address a longstanding unmet clinical need across the globe.
Chronic venous disease affects nearly...
Nicolas J Mouawad (McLaren Health System, Bay City, USA) urges vascular surgeons to “get out of their comfort zone” and become more involved in pulmonary embolism (PE) care.
With over one million cases of deep vein thrombosis (DVT) and/or PE...
The Society for Vascular Surgery (SVS), American Venous Forum (AVF), and American Vein and Lymphatic Society (AVLS) have released the second and final part of new guidelines for the management of varicose veins of the lower extremities. The recommendations,...
Venous News 15—September 2023
Issue highlights:
Sixty years in the making: Deep venous valve technologies set to address “large unmet need worldwide”
Spring/summer 2023 conference round-up: Highlights from the Charing Cross (CX) Symposium, International Vein Congress (IVC), European Venous Forum (EVF)...
In this issue:
Sixty years in the making: Deep venous valve technologies set to address “large unmet need worldwide”
Spring/summer 2023 conference round-up: Highlights from the Charing Cross (CX) Symposium, International Vein Congress (IVC), European Venous Forum (EVF) and more
...
In this issue:
Sixty years in the making: Deep venous valve technologies set to address "large unmet need worldwide"
Spring/summer 2023 conference round-up: Highlights from the Charing Cross (CX) Symposium, International Vein Congress (IVC), European Venous Forum (EVF) and...
“The median thrombus age of DVT patients treated in our centre is 14 days – says Dr Andrew Wigham from Oxford University Hospitals. – We know that traditional treatment options such as thrombolytics and other thrombectomy devices are less...
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has designated three new approved bodies to increase the country’s capacity to certify medical devices.
TÜV SÜD, Intertek, and TÜV Rheinland UK join the four current UK Approved Bodies, almost doubling...
Medtronic has announced that an updated ClosureFast radiofrequency ablation (RFA) catheter in a lower 6Fr profile is now available in the USA following 510(k) clearance from the US Food and Drug Administration (FDA). The ClosureFast procedure is intended to...
“Everything depends on the symptoms,” Aleksandra Jaworucka-Kaczorowska, of the Center of Phlebology and Aesthetic Medicine and the Center of Surgery, Gynaecology and Obstretics in Gorzów Wielkopolski, Poland, stresses in an interview on pelvic venous incompetence with Venous News.
“Your decision...
In a recently published study, dedicated venous stents performed well through pregnancy and postpartum, and a protocol including the use of low-dose antiplatelets in combination with anticoagulation at either a prophylactic or therapeutic dose depending on the patient’s risk...
Researchers report that inferior vena cava (IVC) filter placement position relative to the level of the most inferior renal vein was not associated with differences in IVC thrombosis in a recent single-centre cohort study.
Additional key findings from the study...
This advertorial, sponsored by Inari Medical, is only available in selected countries and geographies.
During a recent webinar hosted by Inari Medical, a multidisciplinary group of physicians focused in on how to select eligible patients for the endovascular treatment of...
This advertorial is sponsored by Inari Medical
“We need to eliminate symptoms as fast as possible—it is not OK just to make things a little better,” says Rick de Graaf (Clinical Centre of Friedrichshafen, Friedrichshafen, Germany), setting out why intervention...
Five-year results of the LAMA randomised controlled trial (RCT) show that both mechanochemical ablation (MOCA) and modern endovenous laser ablation (EVLA) technology are associated with low procedural and post-procedural pain, while clinical outcomes in the short and medium term...
The Society of Interventional Radiology (SIR) has published a position statement offering recommendations on the management of chronic iliofemoral venous obstruction with endovascular placement of metallic stents. The statement, published online in the Journal of Vascular and Interventional Radiology, is a...
Uncertainty underlying the magnitude of risk posed by long distance air travel in venous thromboembolism (VTE) patients has created the need for a deeper, systematic dive into the guidelines and resources providers should be turning to when managing their...
In an interview with Venous News at this year’s Leipzig Interventional Course (LINC 2023; 6–9 June, Leipzig, Germany), Raghu Kolluri (Columbus, USA) outlined the “vast list” of options that are now available for the treatment of patients who require...
Why is clot removal crucial for patients suffering from DVT? What are the major benefits of a lytic-free thrombectomy? Watch Michael Lichtenberg (Arnsberg, Germany) present the outcomes of the Arnsberg ClotTriever Study which confirms the safety and efficacy of...
Despite being considered “generally safe”, cyanoacrylate can cause local reactions in up to 25% of patients, disproportionately affecting women, Asian race and thin patients with a body mass index of <22. This was the conclusion drawn by Eduardo Silva...
Delivering results and recommendations from a systematic review and meta-analysis of novel oral anticoagulants (NOACs) versus low molecular weight heparin (LMWH) in the prevention of venous thromboembolism (VTE) recurrence in cancer patients, Patricia Noreen Bueno (St Luke’s Medical Center,...
Biolitec recently announced that it has extended its ELVeS Radial laser system for the minimally invasive laser treatment of insufficient veins by two further new developments.
The ELVeS Radial 2ring Pro laser fibre is capable of removing even highly...
A study of two risk assessment models (RAMs) for predicting the bleeding risk in patients considered for pharmacologic prophylaxis to prevent venous thromboembolism (VTE) has found that though an increasing risk score correlated with higher bleeding rates, both models...
In a second compassionate use case, interventional vascular surgeon Stefan Stalhoff of Klinikum Hochsauerland (Arnsberg, Germany) used VeinWay's Traversa for venous recanalization to save a patient's arm, with supervision by VeinWay scientific advisory board member Michael Lichtenberg. The patient had...
Inari Medical today announced the launch of two new purpose-built products, the RevCore thrombectomy catheter, and the Triever16 Curve catheter.
According to a company press release, RevCore is the first mechanical thrombectomy device designed to address venous in-stent thrombosis, an...
Gore has announced that the first US patient has been enrolled in a prospective, non-randomised, multicentre, single-arm study with five-year follow-up to evaluate the investigational Gore Viafort vascular stent for the treatment of symptomatic iliofemoral venous obstruction.
The first US...
The US Food and Drug Administration (FDA) has released draft guidance with updated recommendations for good clinical practices (GCPs) aimed at modernising the design and conduct of clinical trials.
In a statement, the regulator said that the updates are intended...
Medtronic has announced that Ken Washington has been appointed chief technology and innovation officer.
Washington joins Medtronic from Amazon where he served as vice president and general manager of consumer robotics, and will lead technology development across industries including robotics,...
“I see a future where this technology may be used to treat people early and avoid the devastating long term consequences of venous insufficiency” Ramon Varcoe (Sydney, Australia) opined in a CX Vascular Live discussion with Erin Murphy (Charlotte,...
Penumbra has announced the US Food and Drug Administration (FDA) clearance and launch of Lightning Bolt 7, which the company claims is the most advanced and powerful arterial thrombectomy system on the market.
Lightning Bolt 7 introduces a new method...
Speaking on the FLAME, FLASH and PEERLESS trials that each collected data on the FlowTriever (Inari Medical) device for the treatment of pulmonary embolism (PE), Ripal Gandhi (Miami Cardiac & Vascular Institute & Miami Cancer Institute, Miami, USA) elaborated...
Presenting “remarkable results” from the JETi registry—a prospective, multicentre, observational study which collected real-world data on the safety, performance and clinical benefits of the Jeti peripheral thrombectomy system—speaker Mahmood K Razavi (Children’s Health of Orange County, Orange, USA) relayed...
Steven Abramowitz (Washington DC, USA) explains the significance of the CLOUT registry—the only registry capturing data on mechanical thrombectomy in deep venous thrombosis (DVT). CLOUT confirms excellent safety results and effectiveness of the ClotTriever system in real-world DVT patients....
This article was provided by the American Vein & Lymphatic Society.
The UIP 2023 World Congress (17–21 September, Miami Beach, USA) will bring professionals from different countries and regions to share knowledge, exchange ideas, and discuss the latest advances in...
Six-month outcomes from the FLASH registry have shown that patients with pulmonary embolism who were treated with mechanical thrombectomy showed significant improvement in symptoms, quality of life and cardiac functions.
The findings were presented as late-breaking clinical research at the...
A recent study concludes that non-thrombotic iliac vein lesion (NIVL) patients have better primary patency after venous stenting than patients with venous thrombotic disorders. Olivier Espitia (CHU de Nantes, Nantes, France) and colleagues report this main finding from a...
Venous disease care has a problem with optics in the context of growth in the number of venous procedures and the spectre of inappropriate care, the 2023 Charing Cross (CX) International Symposium (25–27 April, London, UK) heard.
The conversation thread...
The deep venous consensus update session at the 2023 Charing Cross (CX) International Symposium (25–27 April, London, UK) covered a range of key topics in the field, including issues associated with pelvic venous lesions.
The session opened with a series...
A recent multicentre, prospective study has found that stent deformations are greater in the common iliac vein with higher levels of hip flexion, as well as in iliofemoral veins with hyperextension at the superior ramus of the pubis. The...
This advertorial is sponsored by Merit Medical.
The ClariVein OC endovenous occlusion catheter (Merit Medical) is associated with a good occlusion rate, comparable with other techniques including thermal, without major complications. This is according to a five-year Italian experience with...
Akura Medical announced today it has initiated its first-in-human clinical study of the Akura mechanical thrombectomy platform. A press release notes that the Akura platform is a low-profile solution designed to easily access and efficiently remove large-volume, mixed-morphology clots,...
Cook Medical recently announced that the first patient has been treated in a clinical study to evaluate a new venous valve designed for treating chronic venous insufficiency (CVI). The patient was treated by principal investigator Mauricio Alviar (Clinica de...
“Superficial venous treatments have come a long way in the last 20 years,” Manj Gohel (Cambridge, UK) tells Venous News, before outlining the key aims of the VenaSeal Spectrum programme—which includes three studies (two randomised and one prospective observational...
The Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) application for the VEINRESET multicenter pivotal study that will evaluate Sonovein high-intensity focused ultrasound (HIFU) treatment for varicose veins, it has been announced.
Antonios Gasparis, MD, director...
The CX 2023 Venous & Lymphatic programme it set to be a “highlight” of this year’s meeting, CX co-chair and executive board member Erin Murphy (Sanger Heart and Vascular Institute, Atrium Health, Charlotte, USA) tells Vascular News.
This year’s programme...
UK-based medical device manufacturer Sky Medical Technology today announced the International Wound Journal has published a multicentre randomised self-controlled trial (RCT) from the company.
The study compared standard of care with and without the Geko device in patients with hard-to-heal venous...
W L Gore & Associates (Gore) has announced that the first US patient has been enrolled in a prospective, non-randomised, multicentre, single-arm study with five-year follow-up to evaluate the investigational Gore Viafort vascular stent for the treatment of symptomatic...
Recruitment proved to be a major challenge for the STEVECO (Stent versus conservative treatment in patients with deep venous obstruction) randomised controlled trial (RCT), prompting discussion on how best to randomise patients in future trials, the ethics of doing...
A total of £10 million has been awarded to the Medicines and Healthcare products Regulatory Agency (MHRA)—an executive agency of the UK Department of Health and Social Care (DHSC)—to help bring innovative new medicines and medical technologies to UK...
An analysis of venous stent usage trends in the USA from 2014 to 2021 showed a “significant increase” in stents per day placed over time, despite high-profile recalls of two dedicated venous stents from the market.
Those were among the...
The 2023 annual meeting of the American Venous Forum (AVF; 22–25 February, San Antonio, USA) heard the final results from the first US trial of the emerging varicose vein treatment, Sonovein echotherapy (Theraclion), with data showing a 100% technical...
Robert A Lookstein, who is executive vice chair, Diagnostic, Molecular and Interventional Radiology at the Icahn School of Medicine at Mount Sinai Hospital (New York, USA) today presented the results of a subanalysis of Thrombolex’s National Heart, Lung and...
Speaking to Venous News at VEITHsymposium 2022 (15–19 November, New York, USA) Armando Mansilha (Porto, Portugal) answers the “challenging question” of how to best approach selecting an appropriate care pathway for patients with superficial venous disease. Offering a range...
The European Union’s Council of Ministers has today adopted a resolution to extend the deadline for the certification of medical devices under the Medical Devices Regulation (MDR).
Producers of medical devices will have until 31 December 2027 for higher risk...
Large bore mechanical thrombectomy with the FlowTriever system (Inari Medical) in patients with high-risk pulmonary embolism (PE) was associated with a significantly lower occurrence of meaningful in-hospital adverse clinical outcomes compared to other contemporary treatments, data presented at the...
Among 61 high-risk pulmonary embolism (PE) patients followed through to the 30-day visit in the US cohort of the FLASH registry, no mortalities were recorded, while at 48 hours post-treatment with the FlowTriever mechanical embolectomy system (Inari Medical), likewise,...
Viz.ai has announced it will use its Viz Recruit platform to optimise patient enrolment for the National Institutes of Health (NIH)-funded Pulmonary embolism—thrombus removal with catheter-directed thrombolysis (PE-TRACT) clinical trial.
The company claims that PE-TRACT will be the most rigorous...
In a first-in-human, compassionate-use case approved by the US Food and Drug Administration (FDA), University of Michigan Health (Ann Arbor, USA) interventional radiologists David M Williams and Minhaj S Khaja successfully used VeinWay's Traversa for venous recanalisation to save...
Interim one-year outcomes from the multicentre, prospective, single-arm CLOUT registry investigating use of the ClotTriever thrombectomy system (Inari Medical) in all-comer patients with deep vein thrombosis (DVT) demonstrated that 93.5% of limbs had flow present, 97.1% were compressible and...
Researchers in Colombia behind the first-in-human study of a novel bioprosthetic venous valve designed to treat chronic venous insufficiency (CVI) reported three-year results among the eight remaining patients during the 2023 annual meeting of the American Venous Forum (AVF;...
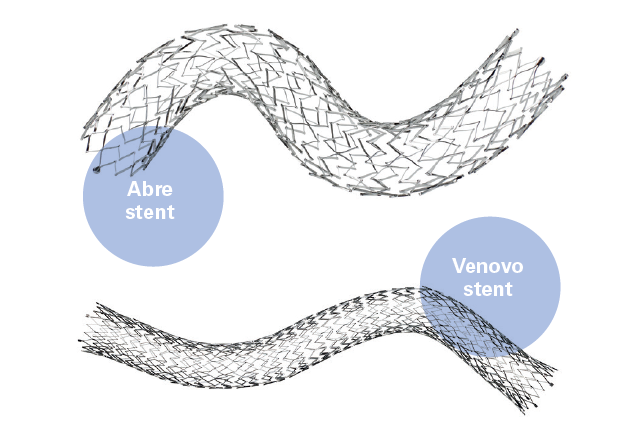
Stephen Black (London, UK) talks to Venous News about the key 36-month results from the ABRE study, which evaluated the safety and effectiveness of the Abre venous self-expanding stent system for the treatment of symptomatic iliofemoral venous outflow obstruction in patients with...
Few adverse events are connected to the use of inferior vena cava (IVC) filters to help prevent deep vein blood clots from developing into pulmonary embolisms (PEs), according to the findings of the Predicting the Safety and Effectiveness of...
In a prospective study of patients undergoing great saphenous vein (GSV) ablation, researchers found no correlation between maximum reflux time and symptom severity as measured by Venous clinical severity score (VCSS).
Authors Damianos G Kokkinidis (Yale University, New Haven, USA)...
NOTE: This video is ONLY available to watch in selected countries and geographies
Iliac side branch devices (ISBs) are “gamechangers in infrarenal therapy of abdominal aortic aneurysms (AAAs),” Mario Lescan (Tübingen, Germany) tells Vascular News. Lescan states that ISBs “allow...
Prominent venous disease experts discuss venous stenting, appropriate care, and the pursuit of refined data and better education in a space where part of the problem involves practitioners moving “freely from being able to do arterial intervention and suddenly...
Theraclion has announced the results of the first Sonovein trial in the USA, which the company describes as a "major milestone" towards receiving US Food and Drug Administration (FDA) approval and accessing the US varicose veins market.
Theraclion reports a...
Inari Medical has announced that the first patient has been enrolled in DEFIANCE, a prospective randomised controlled trial (RCT) comparing the clinical outcomes of patients with iliofemoral deep vein thrombosis (DVT) treated with the ClotTriever system versus anticoagulation only....
Today, Penumbra announced the US Food and Drug Administration (FDA) clearance and launch of its Lightning Flash mechanical thrombectomy system.
"Lightning Flash features Penumbra's novel Lightning intelligent aspiration technology, now with dual clot detection algorithms," the company notes in a...
Koya Medical recently announced the appointment of Thomas Maldonado (New York University Medical Center, New York, USA) as chief medical officer and a member of the company's clinical advisory board.
A press release notes that Maldonado has been one of New York...
An evaluation of the Vascular Quality Initiative’s (VQI) Varicose Vein Registry (VVR) carried out by the Midwestern Vascular Surgical Society (MVSS) has found women to benefit similarly from endovenous ablation as men, but experience fewer complications post-procedure.
The MVSS...
A meta-analysis is first to report the pooled risk of post-thrombotic syndrome (PTS) after isolated distal deep vein thrombosis (DVT). Researchers revealed a one in five risk of long-term PTS after isolated distal DVT, with one in 50 patients...
Corindus has been rebranded to Siemens Healthineers Endovascular Robotics and will sit as a dedicated business within the Advanced Therapies area of Siemens Healthineers, the company has announced.
This brand unification is the final step of the company integration process...
Boston Scientific has announced that it will make a partial offer to acquire a majority stake, up to a maximum of 65%, of shares of Acotec Scientific, a Chinese medical technology company that offers solutions designed for a variety...
Head-to-head observational analysis showed Xarelto as effective in treating cancer-associated thromboembolism (CAT) as apixaban.
The Janssen Pharmaceutical Companies of Johnson & Johnson have announced observational data from eight years of clinical practice showing that the oral Factor Xa inhibitor Xarelto...
Europe’s health commissioner, Stella Kyriakides, has announced that proposals to extend the transition period for the implementation of the European Union’s (EU) Medical Device Regulation (MDR) will be put forward in early 2023.
Kyriakides informed health ministers from the EU’s...
The Society for Vascular Surgery (SVS) has published an update to the SVS/American Venous Forum (AVF) 2011 clinical practice guideline on the care of patients with varicose veins. This guideline update was developed in collaboration with the AVF and...
The Swiss Federal Assembly has voted in favour of accepting medical devices with US Food and Drug Administration (FDA) marketing authorisation in Switzerland.
A motion for ‘more freedom of action in the procurement of medical products for supply of the...
Positive long-term, three-year observational data from a cohort of patients that participated in the previously concluded VenoValve (Envveno Medical) first-in-human clinical trial were recently presented at the VEITHsymposium 2022 (15–19 November, New York, USA).
Principal investigator Jorge Hernando Ulloa (University of...
This advertorial is sponsored by Bentley.
Launched in July 2020, the BeYond Venous self-expanding stent system (Bentley InnoMed GmbH) now has early clinical data and expert experience to support its use in venous interventions. In what Michael Lichtenberg (Arnsberg Vascular...
The Society of Interventional Radiology (SIR) published a position statement deeming endovascular thrombus removal “an acceptable treatment option in selected patients with acute iliofemoral deep vein thrombosis .” The position statement is published online on the Journal of Vascular and...
A retrospective review, presented as a late-breaking clinical trial at The VEINS (Venous Endovascular Interventional Strategies) 2022 (30–31 October, Las Vegas, USA), indicates that single-session thrombectomy for the treatment of iliofemoral deep vein thrombosis (DVT) is associated with reduced...
Two datasets presented during the late-breaking clinical trials session at The VEINS (Venous Endovascular Interventional Strategies) 2022 (30–31 October, Las Vegas, USA)—the latest results from CLOUT and a propensity score-matched analysis of CLOUT versus ATTRACT—bolster the evidence base for...
Gerd Grözinger (Tübingen, Germany) chats with Bernhard Gebauer (Berlin, Germany) at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) 2022 annual meeting (10–14 September, Barcelona, Spain) about some of the difficulties associated with treating patients with pulmonary embolism...
W L Gore & Associates (Gore) today announced the first implants of its investigational Gore Viafort vascular stent as part of the recently initiated Gore Viafort device pivotal clinical study for the treatment of inferior vena cava (IVC) occlusive...
Theraclion announced at the American Vein and Lymphatic Society (AVLS) 2022 annual meeting (13–16 October, New Orleans, USA) that the final patient has been enrolled and treated in the first US study of the company's Sonovein solution for varicose...
Medtronic has announced the 36-month final results from the ABRE clinical study. The purpose of the ABRE clinical study was to evaluate the safety and effectiveness of the company's Abre venous self-expanding stent system, intended for the treatment of...
Tilting or hooking occurred significantly less often in the process of retrieving the Denali inferior vena cava (IVC) filter (BD) than with the Option IVC device, a retrospective review at a tertiary care centre has established.
The research team behind...
John White (Chicago, USA) talks to Venous News at this year’s European Society for Vascular Surgery annual meeting (ESVS 2022; 20–23 September, Rome, Italy), about a procedure that is designed to help treat women who suffer from chronic pelvic...
Results of the FLASH registry demonstrate the “excellent safety profile” of the FlowTriever system (Inari Medical) in 800 “real-world” patients. This is according to Catalin Toma (University of Pittsburgh Medical Center, Pittsburgh, USA), who presented outcomes for the full...
Penumbra and Asahi Intecc, a Japanese medical device manufacturer, announced that they will collaborate to introduce Penumbra’s Indigo aspiration system into the Japanese market upon regulatory approval.
“By bringing together our newest innovations with Asahi’s leadership and expertise in the...
Thrombolex today presented the final results of its National Institutes of Health (NIH)-sponsored RESCUE trial at TCT 2022 (16–19 September, Boston, USA).
This investigational device exemption (IDE) trial demonstrated that pharmacomechanical catheter-directed thrombolysis (PMCDT) therapy using the Bashir endovascular...
The International Consortium for Health Outcomes Measurement (ICHOM) venous thromboembolism (VTE) working group has developed a standard set of outcome measures for patients with VTE. The consensus recommendation—published in the September edition of The Lancet Haematology—is designed to “facilitate...
Bentley today announced that it has acquired the rights of the GoBack catheter from Upstream Peripheral Technologies.
“The acquisition of the GoBack catheter marks the start of inorganic growth for Bentley,” said Sebastian Büchert, Bentley's CEO. “We launched our first...
This advertorial, sponsored by Inari Medical, is only available in selected countries and geographies.
“I am finally very confident I have a device that takes out all the thrombus,” stated Rick de Graaf (Clinical Centre of Friedrichshafen, Friedrichshafen, Germany)...
Viz.ai recently announced it has received US Food and Drug Administration (FDA) 510(k) clearance for an automated right ventricle (RV)/left ventricle (LV) ratio algorithm, a new component of the Viz pulmonary embolism (PE) solution.
According to a company press release,...
Andrew Wigham (Oxford, UK) and Rick De Graaf (Friedrichshafen, Germany) discuss the current state of deep vein thrombosis (DVT) treatment at the Leipzig Interventional Course (LINC) 2022 (6–9 June, Leipzig, Germany), with both noting that “a massive problem” in...
A multicentre, prospective, randomised study published in the Journal of Vascular Surgery: Venous and Lymphatic Disorders (JVS-VL) showed that inspiratory muscle training (IMT), in addition to compression therapy, modified disease activity in patients with chronic venous insufficiency (CVI) with...
Treatment of critically ill COVID-19 patients with full-dose anticoagulation lowers the risk of venous and arterial clotting complications by 44% compared with the standard dose, according to late breaking research presented in a Hot Line session at ESC Congress...
Inari Medical has announced planned enrolment of the DEFIANCE randomised controlled trial (RCT), which is designed to compare the clinical outcomes of patients with iliofemoral deep vein thrombosis (DVT) treated with the ClotTriever system versus anticoagulation only.
The trial will...
Ramona Gupta (Northwestern University, Chicago, USA) addresses the issue of recurrent varicose veins, highlighting in particular the “multiple tools” now at physicians’ disposal to treat the problem.
Superficial venous insufficiency and varicose veins affect approximately 23% of adults worldwide. Treatments...
New data indicate that venous stent failure “may be predicted by low peak flow velocity and post-thrombotic changes in inflow veins” and that endovascular venous stenting for chronic outflow obstructions is an “efficacious and safe” treatment in selected patients.
These...
A new study highlights key differences in clinical features and comorbidities, as well as short-term and also long-term outcomes for patients with distal deep vein thrombosis (DVT) versus proximal DVT. The findings were recently published in JAMA Cardiology.
The differences...
Postprocedural compression of one to two weeks after superficial venous incompetence (SVI) treatment is associated with reduced pain compared with a shorter duration. This is according to a study published in the August edition of the British Journal of...
This week Inari Medical announced that chief operating officer Drew Hykes will succeed Bill Hoffman as chief executive officer, effective 1 January 2023. Hykes will join Inari’s board of directors and Hoffman will also continue to serve on the...
Based on the findings of a multicentre, randomised controlled trial, researchers have concluded that a new iliac vein stent—the Venastent (Tianhong)—provides a “safe and effective” endovascular treatment option for non-thrombotic iliac vein lesions (NIVLs) and is “as efficient as”...
In a prospective, controlled clinical trial of deep vein thrombosis (DVT) triage using artificial intelligence (AI)-guided software simulating compression ultrasonography, lead investigator Efthymios Avgerinos (University of Athens, Athens, Greece) and colleagues demonstrated a high sensitivity and specificity in DVT...
Thrombolex has announced that its board of directors has named Michael Cerminaro chief executive officer (CEO) effective 13 July 2022, the date of the company’s most recent quarterly board meeting.
The company advises Cerminaro will also retain his current title...
Biolitec recently announced the launch of a new addition to its ELVeS Radial family—the ELVeS Radial 2ring Swift fibre. According to a company press release, both larger truncal veins and smaller side branches and perforating veins can be treated...
In a new subgroup analysis of the EXTRACT-PE trial, the Indigo aspiration system (Penumbra) was effective at improving clinical outcomes for submassive pulmonary embolism (PE) patients regardless of emboli location. In addition, clot burden was significantly reduced in both...
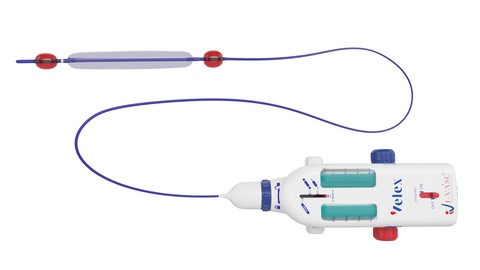
I-Vasc, which has developed and CE marked its percutaneous Velex system to treat chronic venous insufficiency (CVI), has announced that Velex has entered clinical use, with the first two cases performed last week in Milan, Italy, at the Policlinico...
Despite greater comorbid conditions, patients with obesity benefited as much as patients with normal body mass index (BMI) from iliac vein stent placement for proximal venous outflow obstruction (PVOO) in a study from a research group in New York....
Optimed recently announced the presentation of two prospective, multicentre trials evaluating the performance and effectiveness of their venous stent systems at the Leipzig Interventional Course (LINC) 2022 (6–9 June, Leipzig, Germany).
The TOPOS trial two-year results for the Sinus-Obliquus stent...
Ten-year follow-up of a randomised controlled trial (RCT) has shown no clear long-term advantage of endovenous laser ablation (EVLA) with a 980nm wavelength and bare-tip fibre over high ligation and stripping of the great saphenous vein (GSV) under local...
Patients who have suffered from a first episode of unprovoked venous thromboembolism (VTE) have an eight times higher risk of recurrence if they stop treatment after repeated negative D-dimer tests, instead of continuing with low-dose anticoagulant (apixaban), according to...
RapidAI announced today that it has received US Food and Drug Administration (FDA) 510(k) clearance for its Rapid PE Triage & Notification product for fast identification and communication of suspected central pulmonary embolism (PE).
According to a company press release,...
This advertorial is sponsored by Merit Medical. A downloadable version can be found here.
In this case series, Michael Tal (Jerusalem, Israel) explains how the ClariVein OC device (Merit Medical) offers a safe treatment option for venous leg ulcers.
Venous...
This advertorial is sponsored by Merit Medical.
In this case series, Michael Tal (Jerusalem, Israel) explains how the ClariVein OC device (Merit Medical) offers a safe treatment option for venous leg ulcers.
This advertorial is sponsored by Cook Medical. A downloadable version can be found here.
The Zilver Vena venous self-expanding stent system (Cook Medical) is associated with high rates of patency by ultrasound, freedom from clinically-driven reintervention and clinical improvement among...
This advertorial is sponsored by Cook Medical.
The Zilver Vena venous self-expanding stent system (Cook Medical) is associated with high rates of patency by ultrasound, freedom from clinically-driven reintervention and clinical improvement among various challenging subgroups, according to newly released...
BD recently announced that its Venovo venous stent is back on the US market following a recall last year.
In 2019, the company reported that the US Food and Drug Administration (FDA) had granted premarket approval for the Venovo venous...
In an initial cohort of patients enrolled in the CLEAR-DVT study, contemporary venous intervention resulted in an open vein, which reduced post-thrombotic syndrome (PTS). This is the main concluding finding of a Podium 1st presentation delivered by Mitchell Silver...
Magneto Thrombectomy Solutions (Magneto), a medical devices company developing thrombectomy solutions for the treatment of ischaemic stroke and pulmonary embolism, presented successful first-in-human (FIH) results showing safety and feasibility of the eTrieve system, a catheter based on electric fields...
To combat the “overtreatment problem” in the appropriate care of venous disease, “a concerted, complex, multimodal effort” is required from specialists across disparate parts of the world.
That was the conclusion delivered by Manjit Gohel (Cambridge University Hospitals, Cambridge, UK)...
Medtronic has announced that Laura Mauri has been appointed as the company’s chief scientific, medical and regulatory officer. This appointment adds to Mauri's prior responsibilities as chief clinical and regulatory officer, aligning and integrating the company's scientific, medical, clinical research...
Koya Medical recently announced the US commercial availability of its Dayspring active compression system for the treatment of lymphoedema and venous diseases in lower extremities. The company also announced that the Centers for Medicare & Medicaid Services (CMS) issued...
Penumbra has announced that its Indigo aspiration system with Lightning 7 and Lightning 12 have secured CE mark and are now commercially available in Europe. Both technologies are part of Penumbra’s Indigo aspiration system—now with Intelligent Aspiration for mechanical...
Looking to the future, genetic testing may play a role in guiding the intensity of therapeutic interventions and preventive strategies in patients with venous disease. This finding comes from a case-control study on the clinical implications of genetic variation...
I-Vasc, developer of the Velex device with its empty vein ablation (EVA) technology for the treatment of chronic venous insufficiency (CVI), has announced the completion of its Series A financing round.
In addition to the €750,000 tranche raised in the...
A study from Sweden published by The BMJ recently finds an increased risk of deep vein thrombosis (DVT) up to three months after COVID-19 infection, pulmonary embolism (PE) up to six months, and a bleeding event up to two months.
The...
Cordis has announced a strategic investment venture that will expand the scope of the global cardiovascular technology company into the venous thromboembolism (VTE) market with a strategic investment in Endovascular Engineering (E2), a developer of next-generation thrombectomy devices to...
Anticoagulants alone are associated with a high rate of resolution of pulmonary embolism (PE) in patients with acute PE and COVID-19 infection. This was the main concluding finding of a study published in the Journal of Vascular Surgery: Venous...
Sky Medical Technology has announced it has achieved further US Food and Drug Administration (FDA) 510(k) clearance to market the new (W3) geko device variant, for increasing microcirculatory blood flow in lower limb soft tissue of patients with venous...
Bert van Meurs, the chief business leader for Image Guided Therapy with Royal Philips, speaks to Venous News following the company’s acquisition of Vesper Medical in December last year. He details how the procurement will further expand Philips’ portfolio...
In a secondary analysis of the SUNSET sPE randomised controlled trial, Efthymios Avgerinos (University of Pittsburgh Medical Centre, Pittsburgh, USA) and colleagues found differences in biomarker levels in response to treatment with catheter-directed therapies compared to anticoagulation alone. These...
Venous stenting was a hot topic on the agenda of the recent American Venous Forum (AVF) annual meeting (23–26 February, Orlando, USA). Catching the audience’s attention, one presenter highlighted a head-to-head study of dedicated versus non-dedicated stents.
“Iliofemoral venous obstruction when...
Medtronic has announced that it has entered into a contract with Vizient to add Touch Surgery Enterprise, an AI-powered surgical video management and analytics platform for the operating room (OR), to Vizient's offerings.
In a press release, Medtronic said that...
Vascular Breakthroughs and Vascular Care Connecticut have announced the first New England enrolment into the novel DEXTERITY clinical trials of local anti-inflammatory therapy to improve outcomes of deep vein thrombosis (DVT) therapy.
Paul Gagne and the research team at Vascular...
Iliac vein stents tolerate a gravid uterus well and a possible future pregnancy should not contraindicate their usage in the treatment of pelvic venous insufficiency (PVI), according to a recent press release.
According to lead author Peter Pappas (Centre for...
In patients with chronic venous disease (CVD) and associated ipsilateral limb reflux, iliac vein stenting was shown to improve the prevalence of reflux and severity in the long term. This is the main concluding finding of a single-centre retrospective...
An emerging endovenous valve formation system designed to treat patients with chronic venous insufficiency (CVI) with evidence of deep venous reflux has demonstrated continual improvement since US investigators started performing clinical cases with the device 18 months ago, according...
This year’s venous and lymphatic programme at the Charing Cross (CX) International Symposium 2022 (26–28 April, London, UK, in person and virtual) will cover various challenges in the field—ranging from appropriate care conundrums to venous challenges in relation to...
Six-month outcomes from the ongoing CLOUT registry demonstrate the “safety and efficacy” of the ClotTriever thrombectomy system (Inari Medical) in a real-world deep vein thrombosis (DVT) population regardless of clot chronicity level, the 2022 annual meeting of the American...
Charing Cross (CX) chair Roger Greenhalgh welcomes the vascular community to this year's symposium, due to be held 26–28 April in London, UK, and virtually. To read the preliminary programme and register visit cxsymposium.com.
Envveno Medical announced positive 30-month data from the first-in-human trial of the VenoValve bioprosthetic potential venous valve replacement during the 2022 American Venous Forum (AVF; 23–26 February, Orlando, USA).
The results showed that VenoValve recipients—now an average of 30 months...
PolarityTE has announced results from a multicentre randomised controlled trial (RCT) evaluating treatment of venous leg ulcers (VLUs) with its investigational product SkinTE plus standard of care versus standard of care alone. The trial met the primary endpoint of...
Cardiovascular Systems Inc (CSI) recently announced it has partnered with Innova Vascular (Innova) to develop a full line of novel thrombectomy devices.
According to a press release, CSI intends to acquire and commercialise novel thrombectomy devices from Innova targeting peripheral vascular...
In patients with concurrent deep venous obstruction (DVO), superficial venous reflux (SVR) ablation is suggested to be safe, according to a recent systematic review. The authors of the study published in the Journal of Vascular Surgery: Venous and Lymphatic...
Direct oral anti-coagulants (DOACs) work just as well to prevent future clots as warfarin and are less likely to result in major bleeding after a cerebral venous thrombosis (CVT) stroke, according to preliminary late-breaking science presented at the American...
Inari Medical has announced that the first patient has been enrolled in PEERLESS—a prospective, randomised controlled trial (RCT) comparing the outcomes of patients with intermediate-high risk pulmonary embolism (PE) treated with the FlowTriever system versus catheter-directed thrombolysis (CDT).
The first...
Acera Surgical has announced its first patient enrolment in a multicentre, head-to-head clinical study evaluating Restrata for the treatment of non-healing venous leg ulcers (VLUs). William Marston (University of North Carolina School of Medicine, Chapel Hill, USA) is the...
Royal Philips has announced an update to its handheld ultrasound platform—Lumify—adding Pulse Wave Doppler technology to expand the haemodynamic assessment and measurement capabilities of the system.
In a press release, Philips said that the new functionality would enable clinicians to...
Koya Medical has announced the close of a US$26 million Series B financing. The round was led by 3x5 Partners, along with new investors including Asahi Kasei Ventures, Cadence Healthcare Ventures, and existing investors Arboretum Ventures and Scientific Health...
Stent migration following treatment of acute iliofemoral thrombus or venous obstruction is “rare but may be underreported”, with the majority of reported cases being shorter and smaller diameter stents. This is the main concluding finding of a systematic review...
The US Food and Drug Administration (FDA) has issued two final guidances providing recommendations for including patient perspectives in medical device clinical studies.
As per an FDA press release, the finalised version of the first of these two guidance...
A retrospective study of 1,223 iliac vein cases performed in three office-based laboratories (OBLs) shows a major complication rate of 0.41%, suggesting these procedures are safe and well-tolerated in this setting.
“Because OBLs are not regulated to the same degree...
A new study reports that watching TV for four hours a day or more is associated with a 35% higher risk of venous thromboembolism (VTE) compared with less than 2.5 hours. The research is published today in the European...
Deep venous stenting with the Blueflow venous stent (Plus Medica) offers favourable primary patency, and is associated with significant continued improvement of symptom severity in patients with obstructive chronic venous disease (CVD) out to 12 months. Michael Lichtenberg (Vascular...
The Abre venous stent (Medtronic) remains safe and effective for the treatment of iliofemoral venous obstructive disease out to 24 months. This is according to Erin Murphy (Sanger Heart and Vascular, Atrium Health, Charlotte, USA), who presented the latest...
Thrombolex has announced positive results from the prespecified interim analysis of the first 62 evaluable pulmonary embolism (PE) patients enrolled in the investigational RESCUE trial.
RESCUE is a prospective multicentre, single-arm pivotal trial evaluating patient outcomes after treatment of acute...
The European Society for Vascular Surgery (ESVS) has released 2022 clinical practice guidelines on the management of chronic venous disease (CVD) of the lower limb to update its 2015 recommendations. The document, authored by Marianne G De Maeseneer (Erasmus...
The European Society for Vascular Surgery (ESVS) has released 2022 clinical practice guidelines on the management of chronic venous disease (CVD) of the lower limbs to update its 2015 recommendations. The document, authored by Marianne G De Maeseneer (Erasmus...
Mercator MedSystems has recently announced that the DEXTERITY trials research has begun under a technology transfer grant for approximately $300,000 funded by the National Institutes of Health (NIH). Preclinical research performed using the grant resources aims to demonstrate mechanistic...
Akura Medical has announced the closing of its $25M Series A1 financing, which will be used to support the development of its next-generation thrombectomy device.
The financing was led by Cormorant Asset Management, with participation from The Capital Partnership (TCP), PA MedTech...
Venous care in the UK is in a “sorry state at the moment”, Zola Mzimba (Derry, UK) tells Venous News. This is in part down to COVID-19 which has driven a continued rise in waiting lists and patient assessments. However,...
MolecuLight announced today that SWK Holdings has provided US$10 million structured debt to support MolecuLight’s global commercial expansion.
SWK Holdings Corporation is a life science focused specialty finance company catering to small- and mid-sized commercial-stage companies through the creation of...
The Janssen Pharmaceutical Companies of Johnson & Johnson recently announced that the US Food and Drug Administration (FDA) has approved two paediatric indications for Xarelto (rivaroxaban): the treatment of venous thromboembolism (VTE) and reduction in the risk of recurrent...
Vesper Medical recently announced the completion of enrolment in its pivotal study—VIVID (Venous stent for the iliofemoral vein investigational clinical trial using the Vesper Duo venous stent system).
VIVID enrolled its 160th patient earlier this month, marking completion of enrolment...
Sky Medical Technology recently announced US Food and Drug Administration (FDA) 510(k) clearance to market the Geko device for increasing microcirculatory blood flow in lower limb soft tissue of patients with venous insufficiency and/or ischaemia.
According to Sky Medical, the...
Royal Philips has announced US Food and Drug Administration (FDA) de novo clearance for the Philips inferior vena cava (IVC) filter removal laser sheath—CavaClear—to remove an IVC filter when previous methods of removal have failed.
The Philips CavaClear IVC filter...
Royal Philips today announced that it has signed an agreement to acquire Vesper Medical, a US-based medical technology company that develops minimally-invasive peripheral vascular devices.
Vesper Medical will further expand Philips’ portfolio of diagnostic and therapeutic devices with an...
InterVene recently announced that it has received Breakthrough Device designation from the US Food and Drug Administration (FDA) for the company’s BlueLeaf endovenous valve formation system.
According to the company, BlueLeaf is the first catheter-based solution developed for deep vein...
A systematic review and meta-analysis published online in the European Journal of Vascular and Endovascular Surgery has suggested that, following endovenous thermal ablation of varicose veins, compression stockings only slightly reduce pain with no significant improvement to quality of...
The rate of follow-up after an incomplete and negative lower extremity venous duplex ultrasound (I/N LEVDUS) has been shown to increase following a specific recommendation for follow-up in the ultrasound report. This was the main concluding finding of a...
Catherine Arundel (York, UK) talks to Venous News at the UK Vascular Societies’ Annual Scientific Meeting (VSASM 2021; 1–3 December, Manchester, UK) about the VenUS-6 trial—a randomised controlled trial which aims to recruit and randomise 675 people with venous...
BD announced today it has acquired Venclose, a provider of solutions for the treatment of chronic venous insufficiency (CVI).
"We are committed to setting a new standard of excellence for people living with venous disease, and that starts with providing...
A worldwide study led by McMaster University in Hamilton, Canada, has shown that milvexian can be used to prevent venous blood clots with minimal side-effects.
Milvexian is unique in that it works by targeting factor XIa, a clotting enzyme that...
Viz.ai has announced the US commercial launch of its AI-powered modules for pulmonary embolism and aortic disease. Debuting at VEITHsymposium 2021 (16–20 November, Orlando, USA), the new modules allow for faster clinical decision-making and improved care coordination for patients suffering from...
RD Global & Invamed recently announced that five-year results of a comparison between cyanoacrylate ablation (CAA) and radiofrequency ablation (RFA), showing that CAA with the company's VenaBlock venous closure system "seems to be the ideal treatment" for great saphenous...
Medtronic has announced its ambition to achieve net zero carbon emissions by fiscal year 2045 across its operations and value chain to accelerate efforts to combat climate change. The announcement comes amidst the 2021 United Nations Climate Change Conference...
Mercator MedSystems has announced enrolment of the first patient in the DEXTERITY (Dexamethasone therapy examining reduction of inflammation after thrombus removal to yield benefit in DVT) Phase 2 clinical trial. Mercator’s proprietary Bullfrog micro-infusion device is a delivery solution...
A research team led by Lim Chwee Teck from the National University of Singapore’s (NUS; Singapore) Department of Biomedical Engineering and Institute for Health Innovation & Technology (iHealthtech), in collaboration with clinical partners from Singapore General Hospital (Singapore), has...
Inari Medical has announced positive acute and long-term interim results from the first 500 pulmonary embolism (PE) patients enrolled in the FlowTriever outcomes registry (FLASH).
A press release reports that, at 48 hours post procedure, the major adverse event rate...
Envveno Medical, formerly Hancock Jaffe Laboratories, recently announced that the first VenoValve surgery in the company’s SAVVE US pivotal trial for the VenoValve has been successfully completed. The surgery was performed by Adriana Laser, associate professor of surgery at...
Venclose recently announced US Food and Drug Administration (FDA) 510(k) clearance for Venclose Maven, a novel radiofrequency (RF) ablation catheter for minimally invasive treatment of incompetent perforator veins.
“While our existing Venclose RF ablation system, including the proprietary Venclose catheter,...
Three-year results of the VIVO clinical study support the continued safety and effectiveness of the Zilver Vena venous stent (Cook Medical) in the treatment of symptomatic iliofemoral venous outflow obstruction, a press release from the VIVA Foundation summarises. Paul...
Morwan Bahi (Wellington Regional Hospital, Wellington, New Zealand) presented five-year results of a retrospective, observational, single-centre study detailing outcomes for all patients with symptomatic lower limb chronic venous insufficiency treated with saphenous vein closure with VenaSeal (Medtronic) cyanoacrylate ablation...
Nearly half of patients deemed preprocedurally to be suffering an acute case of deep vein thrombosis (DVT) turned out to have a “much more” chronic classification of the condition following intraoperative imaging and thrombus inspection post-procedure, an interim analysis...
Koya Medical recently presented data from the company’s prospective, multicentre, randomised, crossover study comparing Dayspring, the company’s active compression treatment for lymphoedema, to a traditional pneumatic compression pump.
In the trial, study participants reported significantly greater adherence to Dayspring treatment...
Boston Scientific announced positive results for the EkoSonic endovascular system (EKOS system) during a late-breaking clinical trial presentation at Vascular Interventional Advances (VIVA) 2021 (5–7 October, Las Vegas, USA). Data from the KNOCOUT PE registry—established to measure institutional adoption...
Thrombolex recently announced the results of the RESCUE trial’s prespecified interim analysis, of the first 62 evaluable patients, in a late-breaking clinical trials session at Vascular Interventional Advances (VIVA) 2021 (5–7 October, Las Vegas, USA).
This pivotal trial is scheduled...
Preliminary clinical results from a first-in-human study assessing the Amplifi vein dilation system (Artio Medical) were presented at Vascular Interventional Advances (VIVA) 2021 (5–7 October 2021, Las Vegas, USA). Data were presented by Surendra Shenoy—an associate professor of surgery at...
This article is sponsored by the European College of Phlebology.
It is a fact that the incidence of venous disease is rising; currently, 25% of Europe’s population is affected, and this figure will climb to 35% by 2050. Due to...
Koya Medical has received US Food and Drug Administration (FDA) 510(k) clearance for its Dayspring Lite treatment for lymphoedema and venous disease. Unlike traditional compression pumps that require patients to be tethered to the wall during use, the Dayspring...
In a comparison of endovascular versus hybrid treatment of chronic venous obstructions (CVOs) involving the confluence of the common femoral vein, researchers have found that both strategies provide similar patency rates. Writing online in the Journal of Vascular Surgery:...
Inari Medical has announced that the company has appointed Victor F Tapson as vice president of Medical Affairs.
Tapson has devoted his medical career to patient care, research, and teaching in pulmonary hypertension and pulmonary embolism, a press release reads....
A single-centre, prospective, randomised trial suggests that endovenous laser ablation (EVLA) using a 1470nm laser with a bare fibre (BF) provided outcomes in the short term similar to those using a radial fibre (RF).
Endothermal ablation has revolutionised the treatment...
Theraclion has announced US Food and Drug Administration (FDA) approval for the first trial with Sonovein in the USA. After this clinical trial, a full pivotal study will be conducted for FDA review for market authorisation.
The study will be...
LSO Medical has announced CE mark approval of its newest generation endovenous laser therapy (EVLT) SnakeBack assisted system, the Lumeseal laser platform. Designed to enhance ease-of-use and to provide greater precision and control throughout the procedure, Lumeseal is announced...
Today, Boston Scientific announced an agreement to acquire Devoro Medical, developer of the Wolf thrombectomy platform. The non-console and lytic-free Wolf technology targets and captures blood clots using finger-like prongs that retrieve and remove thrombi in the arterial and...
Thrombolex has announced that it will be attending The VEINS (Venous Endovascular INterventional Strategies) conference (Booth T8) and VIVA (Vascular InterVentional Advances) Physicians conference (Booth #502) in Las Vegas, USA from 3–7 October. There will be live demonstrations and...
The progress of two “promising” new devices in the venous valve replacement space were outlined during a special session at the Society for Vascular Surgery’s Vascular Annual Meeting (SVS VAM 2021; 18–21 August, San Diego, USA and online) co-hosted...
“Among patients undergoing vascular surgery, thromboprophylaxis with anticoagulants showed a trend towards reduced incidence of VTE when compared to placebo,” Tarek Haykal (Duke University Medical Center, Durham, USA) and colleagues write in the Journal of Vascular Surgery: Venous...
Following an online-only conference in 2020, this year's iteration of the European Society for Vascular Surgery (ESVS) Annual Meeting will take a hybrid format, with sessions held in Rotterdam, The Netherlands and online from 28–29 September. In this letter,...
Advanced Oxygen Therapy Inc (AOTI) has announced that its multimodality Topical Wound Oxygen (TWO2) therapy was recently highlighted at multiple leading international clinical conferences across the USA and UK.
At this year's Malvern Diabetic Foot Conference (7–9 July, Malvern, UK, the growing...
New research published in Anaesthesia (a journal of the Association of Anaesthetists) shows that venous thromboembolisms (VTEs) are 50% more likely to occur in patients with current COVID-19 infection and almost twice as likely in those with recent infection. The...
The American College of Chest Physicians (CHEST) recently released new clinical guidelines for venous thromboembolism (VTE) management, “Antithrombotic therapy for VTE disease: Second update of the CHEST guideline and expert panel” that provides 29 recommendations on 17 Patients, Interventions, Comparators,...
Hancock Jaffe Laboratories has announced that promising two-year post-VenoValve implantation data are being presented today at the Society for Vascular Surgery’s Vascular Annual Meeting (SVS VAM 2021; 18–21 August, San Diego, USA and online) by Jorge Ulloa (Fundacion Santa...
A new study suggests that the degree of intravascular ultrasound (IVUS)-determined iliofemoral venous stenosis does not appear to affect the initial clinical presentation, clinical-etiologic-anatomic-pathophysiological (CEAP) clinical class, supine foot venous pressure, clinical improvement, quality of life (QoL) improvement, stent...
Koya Medical recently announced that its wearable, active compression therapy system for lymphoedema—Dayspring—has been issued new billing codes by the Center of Medicare & Medicaid Services (CMS) in accordance with the Healthcare Common Procedure Coding System (HCPCS). According to...
Boston Scientific has commenced enrolment in the HI-PEITHO clinical trial, a collaborative research study with the Pulmonary Embolism Response Team (PERT) Consortium and the University Medical Center of the Johannes Gutenberg University of Mainz (Mainz, Germany) comparing use of...
Artio Medical announced today it has completed enrolment in its first-in-human study evaluating the Amplifi vein dilation system. In the study, five patients were treated by Adrian Ebner, head of the Cardiovascular Department at Sanatorio Italiano Hospital in Asunción, Paraguay.
"I am very...
Hancock Jaffe Laboratories today announced that the US Food and Drug Administration (FDA) has granted Breakthrough Device designation status to the VenoValve, the company’s lead product, which is currently set to begin its US pivotal trial.
The VenoValve is a...
A large, single-centre retrospective study has revealed the risk of acute kidney injury (AKI) following pharmacomechanical thrombolysis (PMT) for lower extremity deep vein thrombosis (DVT) is as high as 22%.
PMT is an established treatment for selected patients with...
This advertorial, sponsored by BD, is only available in selected countries and geographies.
In this case report for Venous News, experts outline the importance of recognising chronic venous obstruction as a crucial contributor to non-healing wounds in patients firstly...
Royal Philips today announced the US Food and Drug Administration (FDA) has granted Breakthrough Device designation for a laser-assisted inferior vena cava (IVC) filter removal device. The proposed device is intended for ablating tissue to remove an IVC filter...
This advertorial, sponsored by BD, is only available in selected countries and geographies.
A downloadable version of this advertorial can be found here.
In this case report for Venous News, experts outline the importance of recognising chronic venous obstruction as...
Rachael Morris (London, UK) talks to Venous News about VEINES QoL/Sym—a venous disease-specific quality of life (QoL) questionnaire for patients with chronic venous insufficiency—as well as the findings of a study which sought to evaluate changes in venous-specific QoL...
Transluminal injection of foam sclerotherapy (TLFS) combined with endovenous laser ablation (EVLA) is a safe and feasible procedure that improves venous clinical severity score (VCSS) and reduces additional second-stage interventions compared to ultrasound-guided foam sclerotherapy (UGFS) combined with EVLA....
Based on findings presented at the 21st European Venous Forum (EVF) Annual Meeting (24–26 June, online), Imre Bihari (A+B Clinic, Budapest, Hungary) speaks to Venous News about the benefits of laser crossectomy.
What is the laser crossectomy technique used for...
Viz.ai has partnered with Avicenna.AI to enable intelligent care coordination and improve patient triage of patients suffering from pulmonary embolism (PE) and aortic disease.
Avicenna.AI’s US Food and Drug Administration (FDA)-approved algorithms for PE and type A and type B aortic...
Fist Assist Devices has announced a two-year affiliation with Airos Medical to commercialise and launch sales of the Fist Assist FA-1 device in the USA. The Fist Assist FA-1 is a wearable, patent-protected, intermittent compression device that recently received...
Surmodics recently announced that it has acquired privately-held Vetex Medical Limited. The Galway, Ireland-based medical device developer and manufacturer has focused exclusively on venous clot removal solutions. The transaction expands Surmodics’ thrombectomy portfolio with a second US Food and...
Despite a high rate of initial anticoagulation, patients with isolated superficial vein thrombosis (SVT) are at risk of thromboembolic complications—including recurrent or extended SVT and recurrent venous thromboembolism (VTE)—at three-month follow-up. This is according to the INSIGHTS-SVT (Investigating significant...
Venous disease-specific quality of life (QoL), as measured by the VEINES (Venous insufficiency epidemiological and economic study)-QoL/Sym, improves significantly after iliac vein stenting for chronic venous obstruction. This is according to the 2021 European Venous Forum (EVF) Prize-winning paper...
The US Food and Drug Administration (FDA) has enhanced the indications for use of the Bashir endovascular catheter (Thrombolex) and the Bashir Plus endovascular catheters (Bashir+10, Bashir+20, Bashir+30, Bashir+40; Thrombolex). These catheters are intended for the controlled and selective...
The Janssen Pharmaceutical Companies of Johnson & Johnson recently announced it has submitted a new drug application (NDA) to the US Food and Drug Administration (FDA) for the use of Xarelto (rivaroxaban) in paediatric patients.
The NDA seeks two...
Fist Assist Devices has received US Food and Drug Administration (FDA) 510(k) clearance for use of the Fist Assist FA-1 device in the USA as an arm massage and increased vein circulation device, and has commenced marketing the Fist...
Venous stenting can have myriad benefits for carefully selected patients with deep and pelvic venous disease. However, as venous stenting has quickly gained popularity, concerns have arisen about the appropriateness of its use and the potential complications that may occur.
Rick...
Among patients with a Caprini score of ≥11 who received standard prophylaxis for venous thromboembolism (VTE), adjunctive intermittent pneumatic compression (IPC) resulted in a significantly lower incidence of asymptomatic venous thrombosis.
This is according to the findings of the...
A study by Mayo Clinic researchers provides some clarity on the use of direct oral anticoagulants (DOACs), such as apixaban and rivaroxaban, to treat acute venous thromboembolism (VTE) in patients with gastrointestinal cancers. The findings were published 2 June...
Inari Medical today announced "strongly positive" interim results from the first 250 deep vein thrombosis (DVT) patients enrolled in the CLOUT (ClotTriever outcomes) registry. These latest data showed that the ClotTriever system removed 100% of the blood clots in...
A study suggests avoiding propofol for intraprocedural sedation during catheter-directed interventions (CDIs) for intermediate-risk pulmonary embolism (PE) because it can have detrimental effects. Propofol is the most commonly used parenteral anaesthetic agent in the USA, extensively used for minor...
Medical imaging artificial intelligence (AI) specialist Avicenna.AI recently announced that it has received certification in the USA and European Union (EU) for CINA CHEST, its new AI solution that leverages deep learning algorithms for emergency triage of deadly vascular...
Monroe Carell Jr Children's Hospital at Vanderbilt (Nashville, USA) has launched a study to determine the impact of a predictive model for identifying paediatric patients at risk for developing venous thromboembolism (VTE).
The study uses advanced predictive analytics to inform...
Koya Medical announced today that it has received US Food and Drug Administration (FDA) 510(k) clearance for its active compression therapy system Dayspring for the treatment of lymphoedema and venous diseases that impact lymphatic flow in the lower extremities....
The European Union (EU) Medical Devices Regulation (MDR) takes effect from today (26 May 2021).
The Regulation revises quality and safety standards and the range of regulated devices and was first initiated in May 2017, with an initial three-year...
Boston Scientific and BD have both initiated recalls of venous stents. According to a US Food and Drug Administration (FDA) medical device recall notice posted 21 May, Boston Scientific has recalled its Vici venous stent system (Vici SDS) and...
LimFlow SA recently announced the publication of 12-month data from the full patient cohort in its PROMISE I study of the LimFlow percutaneous deep vein arterialisation (pDVA) system in the Journal of Vascular Surgery.
Results showed sustained positive outcomes...
Manjit Gohel (Cambridge, UK) and Stephen Black (London, UK) write that several recent advances in the management of venous thromboembolism (VTE) have provided clarity and consensus on this important topic. However, they stress that areas of controversy remain.
VTE has...
At VENOUS2021 (17–20 March, virtual), the annual meeting of the American Venous Forum, Geno Merli (Thomas Jefferson University Hospital, Philadelphia, USA) gave a keynote lecture on venous thromboembolism (VTE) in COVID-19 patients, detailing an “acute and extended” risk in...
The publication of positive new data and a need to minimise hospital visits during the COVID-19 pandemic has seen a general trend towards using more direct oral anticoagulants (DOACs), write Ankur Thapar, a consultant vascular surgeon, and Premalatha Sharavanan,...
While various treatment regimens for the “unique complication” of endothermal heat-induced thrombosis (EHIT) following endovenous ablation have been developed, Lowell S Kabnick tells Venous News, none have achieved universal acceptance. That is, he argues, until now. In this article,...
From the first research linking heparin to a reduction in fatal pulmonary embolism (PE), to the development and refinement of risk assessment tools to lower the risk of thrombosis, Joseph Caprini (University of Chicago Pritzker School of Medicine, Chicago,...
Sky Medical Technology has announced the inclusion of its flagship product, the Geko device, on a US Veterans Health Administration (VA) Federal Supply Schedule (FSS) contract awarded to its federal distribution partner, Marathon Medical.
The wearable, clinically proven Geko is...
As evolving treatment modalities offer the potential for improved outcomes in patients with deep venous disease, the importance of patient selection and the need for high-volume expert treatment centres to treat these patients were underscored by world-leading experts during...
An international panel of venous and lymphatic experts has called for a multidisciplinary approach to identify the causes of severely swollen lower limbs and to improve the treatment of this common condition, which has been poorly managed all over...
Hancock Jaffe Laboratories has revealed that the United States Patent and Trademark Office (USPTO) has issued the first patent covering the company's VenoValve. The patent is entitled 'Implantable Vein Frame' and is US patent number 10,959,841.
The company recently announced...
The results of a two-year study support the continued safety and effectiveness of the Zilver Vena Venous Stent (Cook Medical) in treating symptomatic iliofemoral venous outflow obstruction, according to findings presented at the 2021 meeting of the Society of...
Hancock Jaffe Laboratories recently announced that the US Food and Drug Administration (FDA) has approved the company's Investigational Device Exemption (IDE) application to begin the US pivotal trial for the VenoValve. The VenoValve is an implantable valve designed to...
A recent study on iliac vein stenting showed better short- and mid-term patency rates when intravascular ultrasound (IVUS) was utilised prior to stent deployment in addition to multiplanar venography compared to venography alone. The data were presented at VENOUS2021 (17–20...
According to a recent study, patients with cancer under immune checkpoint inhibitor therapy are at high risk of thromboembolism, especially venous thromboembolism (VTE). Furthermore, VTE occurrence was associated with increased mortality. Florian Moik (Comprehensive Cancer Center of MedUni...
Inari Medical has announced the enrolment of the first high-risk pulmonary embolism (PE) patient in the FLAME (FlowTriever for acute massive pulmonary embolism) study. One in 20 PE diagnoses is categorised as high risk and these are associated with...
Faced with a need to limit hospital visits and conserve personal protective equipment (PPE) in the wake of COVID-19, an 11-centre, hospital-based anticoagulation monitoring service (AMS) in Ohio, USA, needed to adapt fast. Addressing viewers of VENOUS2021 (17–20 March,...
A new review concludes that the implementation of clinical guidelines for the management of patients with deep vein thrombosis (DVT) varies among countries, from strict adherence to no adherence at all. “In this content,” suggest authors Carla Rognoni (SDA...
LimFlow announced today that the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) has approved its Clinical Trial Notification (CTN) for the Japanese cohort of the ongoing PROMISE II pivotal trial of the LimFlow percutaneous deep vein arterialisation (pDVA) system.
PROMISE...
Long-term results of patients who underwent outpatient laser treatment for varicose veins at the great saphenous vein (GSV) or small saphenous vein (SSV) in 2008–2009 have been published in the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
The...
Vetex Medical has announced positive one-year outcomes from a European clinical study of the ReVene thrombectomy catheter. In patients with iliofemoral vein thrombus, the device was found to significantly improve symptoms and quality of life, while reducing leg swelling....
A new study has found that patients who require recanalisation of a completely occluded venous outflow tract before stenting have a high rate of early reocclusion. Writing in the Journal of Vascular Surgery: Venous and Lymphatic Disorders, William A...
According to a recent study, longer segments of great saphenous vein (GSV) reflux appear to correlate with symptom severity. In addition, researchers noted a small to moderate correlation between length of GSV segment ablated and symptom improvement, which they...
Lowell Kabnick (Lake Worth, USA) talks to Venous News ahead of this year’s Charing Cross Symposium (CX), which is being held online 19–22 April. In 2021, the conference will focus on controversies within the vascular and endovascular space. Kabnick discusses...
Vetex Medical has announced US Food and Drug Administration (FDA) 510(k) clearance for the ReVene thrombectomy catheter.
ReVene uses dual-action technology designed to de-clot peripheral vessels, such as in deep vein thrombosis (DVT), through wall-to-wall contact in a single...
In a comparison of two clinical scales that assess quality of life (QoL) in post-thrombotic syndrome, Angela Lee (McGill University, Montreal, Canada) and colleagues conclude that, when a single scale is used for this purpose, the Villalta scale will...
In a recent meta-analysis, Yang Liu, Huan Lu (Henan Cancer Hospital, Zhengzhou, China), and colleagues found insufficient evidence to prove that inferior vena cava (IVC) filters can reduce pulmonary embolism (PE)-related mortality and overall mortality. However, they did find...
Results of a first-in-human study to assess the safety and feasibility of the Bashir endovascular catheter (Thrombolex) for the treatment of acute intermediate-risk pulmonary embolism (PE) have been published in Circulation: Cardiovascular Interventions.
Authors Akhilesh Sista (New York University...
The first patient has been enrolled in a postmarket clinical follow-up (PMCF) study of the BeYond venous self-expanding stent system (Bentley), a press release states. The stent was launched in a selection of venous centres across Europe in late 2020.
Michael...
Hancock Jaffe Laboratories recently announced that it has completed a public offering of its securities, generating approximately US$41.4 million of gross proceeds, prior to deducting underwriting discounts and commissions, and expenses. Proceeds from the offering will be used for...
Society for Vascular Surgery (SVS) Vascular Quality Initiative (VQI) venous case volume and registry data entry was “sharply reduced” during the initial phase of the COVID-19 pandemic, a retrospective review has concluded. Findings were published online in the Journal...
Outcomes of the COMETA (Compression following endothermal ablation) study were published in the February edition of Annals of Surgery. The results suggest that wearing compression stockings after endothermal ablation is “advantageous in the first few days after treatment” and...
Bayer has received approval in the UK for the use of its oral Factor Xa inhibitor Xarelto (rivaroxaban) to treat venous thromboembolism (VTE) and to prevent VTE recurrence in children from birth to below 18 years after at least...
Michael Lichtenberg (Karolinen Hospital, Arnsberg, Germany) details three-year data from the Arnsberg venous registry on the VICI Venous Stent System (Boston Scientific).
This video forms part of a digital advertorial sponsored by Boston Scientific.
This advertorial is sponsored by Boston Scientific.
The VICI Venous Stent System (Boston Scientific) is associated with excellent primary patency rates, with concomitant symptomatic improvement in Clinical-Etiological-Anatomical-Pathophysiological (CEAP) classification, directly after stenting and in long-term follow-up out to 36 months....
The VICI Venous Stent System (Boston Scientific) is associated with excellent primary patency rates, with concomitant symptomatic improvement in Clinical-Etiological-Anatomical-Pathophysiological (CEAP) classification, directly after stenting and in long-term follow-up out to 36 months. This is the headline finding of...
“I consider intravascular ultrasound (IVUS) to be essential equipment in venous interventions,” Rick de Graaf (Clinic of Friedrichshafen, Friedrichshafen, Germany) told viewers of LINC 2021 (The Leipzig Interventional Course; 25–29 January, online). De Graaf was speaking during a session...
Control Medical Technology recently announced that their US Food and Drug Administration (FDA)-cleared Control 11F mechanical thrombectomy system has been used to remove large blood clots from patients with deep vein thrombosis (DVT).
“The Control 11 French mechanical thrombectomy system...
A large, prospective, single-centre, randomised trial from Spain comparing two open surgical techniques with radiofrequency ablation (RFA) to treat saphenous incompetence found RFA comparable to both invasive approaches in terms of clinical recurrence and quality of life at two...
Inari Medical has announced strongly positive interim results of the first 64 chronic deep vein thrombosis (DVT) patients enrolled in the ClotTriever outcomes (CLOUT) registry. The subanalysis, which focused on patients with an estimated clot age over six weeks,...
Venous thromboembolism (VTE) in COVID-19 patients was a key topic on the agenda of The VEINS (Venous Endovascular INterventional Strategies) session at VIVA 2020 (Vascular InterVentional Advances; 14–15 November, virtual). Based on emerging data and guidelines, Yogen Kanthi (National...
The nature of venous thromboembolism (VTE) in COVID-19 patients is complex, and treatment strategies regarding drug type and dosing continue to evolve, writes Sanjum Sethi. In this context, Sethi concludes that a multidisciplinary approach to treatment, in the form...
Describing the Northwell CORE-19 registry as “the largest prospective registry of thromboembolic complications in hospitalised COVID-19 patients in the post-hospital discharge period,” lead investigator Alex Spyropoulos (Northwell Health and Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, New...
The European Society for Vascular Surgery (ESVS) has produced a clinical practice guidelines document on the management of venous thrombosis—the first guidelines the society have produced on this topic. The document was published as an Editor’s Choice paper in...
Results from the EXTRACT-PE trial, which found that Penumbra’s Indigo aspiration system met its predefined safety and efficacy endpoints for the treatment of pulmonary embolism (PE), have been published in JACC: Cardiovascular Interventions.
Penumbra’s Indigo system is designed to remove...
A recent randomised controlled trial shows a potential role for higher density types of thermal water in lower limb volume control and quality of life improvement. Investigators Erica Menegatti (University of Ferrara, Ferrara, Italy) and colleagues write in an...
In this article for Venous News, Stephen A Black discusses innovation in the field of thrombolytic-free thrombectomy. He considers "exciting" new developments in mechanical-only approaches and concludes that, "We are making progress on our important objectives as vascular specialists...
The excimer laser sheath technique is safe and effective for removing embedded inferior vena cava (IVC) filters refractory to high-force retrieval, the investigators of a first-in-human escalation trial conclude in the Journal of the American Heart Association (JAHA). “This...
Mahmood Razavi (Orange, USA) speaks to Venous News about the recently initiated VIVID trial – a prospective, multicentre, single-arm study which has just begun enrolment and is designed to evaluate the safety and efficacy of the Vesper DUO Venous Stent System in the...
In a recent randomised controlled trial, an age-adjusted, weight-adjusted dabigatran dosing algorithm was found to be appropriate in children under 18 years old with venous thromboembolism (VTE). Writing in Lancet: Haematology, Jacqueline Halton (University of Ottawa, Ottawa, Canada) and...
In a recent study, factors related with venous thromboembolism (VTE) severity—including pulmonary embolism response team (PERT) referral from the intensive care unit (ICU) and right ventricular dysfunction—and elevated bleeding risk were associated with inferior vena cava (IVC) filter placement...
Aidoc and Imbio have announced a partnership intended to provide an end-to-end artificial intelligence (AI) solution for pulmonary embolism (PE), with the goal of ultimately improving efficiency and quality of PE detection and treatment.
"Achieving the value of AI in clinical...
Vesper Medical recently announced initiation of its US Food and Drug Administration (FDA) investigational device exemption (IDE) study—VIVID (Venous stent for the iliofemoral vein investigational clinical trial using the Vesper Duo venous stent system).
The VIVID trial is a prospective, multicentre, single-arm...
In a study of interface pressure changes under compression bandages, Junjie Ning, Fedor Lurie (both Jobst Vascular Institute, Toledo, USA), and colleagues found that while pressure decreased over time under all studied bandages, the temporal pattern of pressure change...
Cook Medical has announced that their Zilver Vena venous self-expanding stent is now commercially available to physicians in the USA.
Zilver Vena is a self-expanding stent designed to meet the needs of patients suffering from symptomatic iliofemoral venous outflow obstruction....
Penumbra today announced US Food and Drug Administration (FDA) 510(k) clearance for expanded indication of the latest iteration of the Indigo aspiration system, Lightning 12.
As part of the Indigo aspiration system, Lightning 12 (Indigo system CAT 12 aspiration catheter...
Amy Ferris (Geriatric and General Medicine Cardiff, Cardiff, Wales, UK), Keith Harding (Welsh Wound Innovation Centre, Ynysmaerdy, Wales, UK), and colleagues have landed on a “suitable protocol” for measuring serum and wound fluid iron levels in the context of...
Inari Medical has announced follow-up results of the first 230 patients enrolled in its FLASH study. FLASH is a real-world registry to study the FlowTriever system in intermediate- and high-risk pulmonary embolism (PE) patients.
A press release reports that...
Anthony Comerota (Alexandria, USA), global principal investigator of the VIVO trial, discusses the highlights of the 12-month results of the VIVO clinical study that were presented at VIVA 2020.
While the safety and efficacy of the Zilver Vena stent, which...
Vascular Barcelona Devices (VB Devices) recently announced that its Varixio Pod Air product received CE mark approval as a Class 1s medical device. Varixio is a novel, patent-protected system that automates the preparation of foam for sclerotherapy of varicose...
In September 2020, a consensus document outlining the Venous and Lymphatic Triage and Acuity Scale (VELTAS) was co-published in Phlebology and the Journal of Vascular Surgery: Venous and Lymphatic Disorders. The document, authored by representatives of various international societies,...
LimFlow SA has announced the presentation of one-year data from the full patient cohort in its PROMISE I study of the LimFlow percutaneous deep vein arterialisation system, showing sustained positive outcomes for both amputation-free survival and complete wound healing....
Results of the VIVO clinical study support the safety and effectiveness of Cook Medical's recently FDA-cleared Zilver Vena venous stent for the treatment of symptomatic iliofemoral venous outflow obstruction, Anthony Comerota (Inova Fairfax Hospital, Alexandria, USA) revealed in a...
Catalin Toma (University of Pittsburgh, Pittsburgh, USA) recently presented interim results from the FLASH registry during a late-breaking session at TCT Connect (14–18 October, virtual), the 32nd annual scientific symposium of the Cardiovascular Research Foundation (CRF).
FLASH (FlowTriever all-comer registry...
An off-label randomised controlled trial (RCT) has found that an investigational stocking, which has no compression in the foot or heel area, is significantly easier to put on and take off, with no inferiority in oedema prevention, compared with...
Results of the ATLANTIS (Aquatic therapy to lower adverse consequences of venous thrombosis and insufficiency) randomised controlled trial (RCT) show that the addition of aquatic activity to the treatment of patients with advanced chronic venous insufficiency is safe and...
Medtronic today announced it has received US Food and Drug Administration (FDA) approval for the Abre venous self-expanding stent system. This device is indicated for use in the iliofemoral veins in patients with symptomatic iliofemoral venous outflow obstruction.
The FDA...
The BASHIR™ Endovascular Catheter offers a safe and easy-to-use platform technology, creating immediate blood flow, accelerating thrombolysis, and dissolving large volumes of thrombus burden. This educational supplement, sponsored by Thrombolex, explores the use of various catheter-directed therapies, including their...
The BAS (British Association of Sclerotherapists) interactive Masterclass Series kicked off on 8 October with three experts from the world of vascular surgery sharing their knowledge of 2020 varicose vein treatments.
One of the UK’s most experienced vein experts, Bruce...
Cook Medical today announced that the Zilver Vena received US Food and Drug Administration (FDA) premarket approval (PMA) in the USA. The product is expected to be commercially available to physicians in the USA in Q4 2020.
Zilver Vena is...
In a systematic review of the worldwide published data on venous thromboembolism (VTE) in COVID-19 patients, Cihan Ay, Stephan Nopp, and Florian Moik (Medical University of Vienna, Vienna, Austria) provide an in-depth analysis on the risk of VTE in...
Patients with venous thromboembolism (VTE) carry a high risk of recurrence. Accordingly, a 16-year Danish prospective cohort study of nearly 74,000 patients with incident VTE concluded that the risk of recurrence is substantial. Furthermore, the scientists found that the recurrence...
Midterm results from a study of the largest population of patients with no-option chronic limb-threatening ischaemia (CLTI) treated with percutaneous deep vein arterialisation (pDVA) using the LimFlow device show that, in this complex group of patients, this treatment method...
The American Venous Forum (AVF) is to stage a four-part series of educational sessions aimed at residents, fellows, and early-career practitioners in vascular surgery, interventional radiology, and a host of other specialties.
The live, virtual course—taking place at 8 pm...
Royal Philips has announced the launch of the QuickClear mechanical thrombectomy system. The single-use system delivers an all-in-one aspiration pump and catheter to remove blood clots from the vessels of the peripheral arterial and venous systems and has received...
To deliver clinical and cost benefits, leg ulcer care pathways should be revised to include early assessment and treatment of superficial venous reflux, researchers behind a recent Journal of the American Medical Association (JAMA) Surgery study suggest.
The investigators set...
In a recent nationwide study of observational data from the USA, intracranial haemorrhage (ICH) was found to be rare among deep vein thrombosis (DVT) patients treated with catheter-directed thrombolysis (CDT). The study showed that the incidence of ICH has...
A recent systematic review and meta-analysis compared the outcomes of both concomitant and staged superficial varicose tributary (SVT) interventions as an adjunct to endovenous truncal ablation. While both treatments demonstrated safety and efficacy, improvements in early disease severity and...
The Society of Interventional Radiology (SIR) recently published new clinical practice guidelines that provide evidence-based recommendations on the use of inferior vena cava (IVC) filters to treat venous thromboembolism (VTE).
“These guidelines allow physicians treating patients at risk of a...
In a recent analysis of 150 patients with post-thrombotic syndrome included in the Swiss and Arnsberg venous stent registries, braided as compared with laser-cut nitinol stents for common femoral vein obstruction appeared to be associated with favourable primary patency...
A new analysis has found that the incidence of cerebral venous thrombosis (CVT) in the USA is higher than previously reported and has increased over time.
According to an American Academy of Neurology press release, the study found the...
Patients with acute pulmonary embolism (PE) can be selected for home management using the sPESI score or the Hestia criteria, according to results of the HOME-PE trial presented in a Hot Line session at the European Society of Cardiology...
The Venovo venous stent system from BD has been revealed as one of the 2020 50th Annual Prix Galien USA award nominees in the "Best Medical Technology" category. The Prix Galien award is among the global health innovation industry's...
A recently-published study concludes that three-dimensional (3D) computed tomography venogram (CTV) enables accurate diagnosis and treatment of patients presenting with symptomatic chronic iliofemoral venous obstruction (CIVO).
Writing in the Journal of Vascular Surgery: Venous and Lymphatic Disorders, authors Arjun...
The American Heart Association (AHA) has announced that after nearly a decade of steady decline, the death rate for people with pulmonary embolism (PE) reversed course and began rising over the past decade. The study was recently published online...
Three-year results of a randomised controlled trial reveal that, while mechanochemical ablation (MOCA) is a “feasible treatment option” in an outpatient setting, its technical success rates are inferior compared to endovenous thermal ablation. The findings were recently published online...
Thrombolex has announced that it has enrolled the first patient in its pivotal RESCUE trial for the treatment of patients with acute submassive pulmonary embolism (PE) using the Bashir endovascular catheter, under an Investigational Device Exemption (IDE) from the US...
A recent meta-analysis found that direct oral anticoagulants (DOACs) appear to be a “reasonable alternative” to low molecular weight heparin (LMWH) for the treatment of venous thromboembolism (VTE) in cancer patients. The study appeared online on 25 July in...
A randomised controlled trial has found that clinical and patient-reported outcomes following radiofrequency ablation (RFA) of varicose veins with compression are no better than without compression. Madu Onwudike (Manchester University NHS Foundation Trust, Manchester, UK) and colleagues conducted the...
Venous News takes a look back at VIVA 2019 (Vascular InterVentional Advances; 4–7 November, Las Vegas, USA) where Stephen Black (London, UK) and Mitchell Silver (Columbus, USA) discuss the CLEAR-DVT study.
Silver touches on some of the “historic studies” of venous intervention such as ATTRACT and CaVenT and compares them to CLEAR-DVT, which he...
Bentley has announced receipt of the CE mark for its BeYond venous self-expanding stent system. The company will initially launch the stent to an exclusive circle of leading venous experts to gain first-hand experience with this novel device.
The first-in-man...
PolarityTE have announced positive results from a protocol-specified interim analysis of the first 50 patients enrolled in a multicentre randomised controlled trial evaluating treatment of diabetic foot ulcers with SkinTE plus standard of care (SOC) versus SOC alone.
SkinTE is...
The International Society on Thrombosis and Haemostasis (ISTH), on behalf of the international thrombosis community, have called on the World Health Organisation (WHO) to share learning and support a systematic approach for managing venous thromboembolism (VTE) in patients with...
In a study evaluating rivaroxaban (Xarelto; Bayer) versus warfarin for the treatment and prevention of recurrent venous thromboembolism (VTE) in patients with obesity, results favoured rivaroxaban at three, six, and 12 months.
First author Olivia S Costa (University of...
Daiichi Sankyo Europe GmbH has announced results from five analyses of 12-month data from ETNA-VTE (Edoxaban treatment in routine clinical practice in patients with venous thromboembolism), a non-interventional, post-authorisation safety study (PASS) evaluating edoxaban (known by the brand name...
The International Society on Thrombosis and Haemostasis (ISTH) and the American Heart Association (AHA) have released a joint scientific statement identifying the top five priorities for research on venous thromboembolism (VTE); the statement has been published in the ISTH journal Research...
Thrombolex has announced that it was awarded a US$3 million Small Business Innovation Research (SBIR) grant from the National Heart, Lung and Blood Institute (NHLBI) section of the National Institutes of Health (NIH) to fund their pivotal RESCUE trial...
A recent study on iliac vein stenting showed lower long-term patency rates for women when compared to their male counterparts. The data were presented during the Vascular and Endovascular Surgical Society (VESS) session of SVS ONLINE (20 June–2 July)....
Manj Gohel (Cambridge, UK) is joined by a host of venous experts including Beverley Hunt (London, UK), Stephen Black (London, UK) and Ian Franklin (London, UK) to look at the long-term impact of the COVID-19 crisis on the treatment...
A poll of an enthused audience of venous specialists during the CX 2020 LIVE Deep Venous Disease Consensus session found that 91% of them were in favour of using venous stents. Armando Mansilha (Porto, Portugal) chaired the session. Moderator Manj Gohel (Cambridge, UK) subsequently...
Kathleen Gibson (Bellevue, USA) put the five-year data from the VeClose study, which looked at the treatment of patients with venous reflux in the great saphenous vein (GSV) with either the VenaSeal closure system (Medtronic) or radiofrequency ablation (RFA) therapy,...
Six venous specialists, Manj Gohel (Cambridge, UK), Ian Franklin (London, UK) Alun Davies (London, UK), Sriram Narayanan (Singapore), Raghu Kolluri (Columbus, USA) and Stephen Black (London, UK) discuss the role of telehealth during the COVID-19 crisis and beyond and...
Medtronic has announced the first-ever results from the ABRE clinical study assessing the safety and effectiveness of the investigational Abre venous self-expanding stent system in subjects with iliofemoral venous outflow obstruction. The study met the primary safety and effectiveness...
In this issue:
Compression stockings might not be needed to prevent blood clots after surgery (p. 1)
CX 2020 LIVE brings superficial venous thrombosis to the forefront (p. 1)
Paul Gagne considers the future of intravascular ultrasound (IVSU) for...
For the first venous session of CX 2020 LIVE, 759 attendees from 79 different countries tuned in. Each audience member could submit their name, country, and a question or point to the speaker in real-time, enabling interaction, discussion, and...
Transit Scientific has received US Food and Drug Administration (FDA) clearance for the XO Score percutaneous transluminal angioplasty (PTA) scoring sheath platform for use in iliac, ilio-femoral, popliteal, infra-popliteal, and renal arterial plus synthetic and/or native arteriovenous haemodialysis fistula.
Angioplasty...
BD has announced the launch of the Halo One thin-walled guiding sheath, designed to perform as both a guiding sheath and an introducer sheath, for use in peripheral arterial and venous procedures requiring percutaneous introduction of intravascular devices.
According to...
VisionQuest Biomedical and the University of New Mexico School of Medicine have been awarded a three-year US$3 million grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health (NIH),...
New research published in the New England Journal of Medicine shows that the respiratory virus SARS-CoV-2, which causes COVID-19, causes severe damage to blood vessels, leading to widespread thrombosis, a press release by the Angiogenesis Foundation reports.
The study was...
A recent systematic review and meta-analysis showed that, in patients with iliofemoral deep vein thrombosis (DVT), percutaneous mechanical thrombectomy was associated with a higher cumulative six-month primary patency and a lower incidence of major bleeding compared to thrombolysis alone....
Manj Gohel (Cambridge, UK), who is also a member of the CX Venous Executive Board, welcomes you to CX 2020 LIVE, which will include some of the major highlights from the deep and superficial venous programmes.
A number of recently...
Venous thromboembolism (VTE) is the leading cause of preventable hospital death in the USA and worldwide. A new policy statement by the American Heart Association provides a focused review of VTE, risk scoring systems, preventive measures for the hospital...
LimFlow SA today announced publication of positive two-year data from the ALPS registry of the LimFlow percutaneous deep vein arterialisation (pDVA) system. Results were published online yesterday in the Journal of Endovascular Therapy and will also appear in the...
Sky Medical Technology highlights a publication on 5 May 2020 in the Journal of Thrombosis and Haemostasis showing the incidence of venous thromboembolism (VTE) in 198 hospitalised COVID-19 patients. The overall rate was 42% at 21 days including patients in...
This video is only available in selected geographies
Stephen Black (London, UK) talks to VEITHtv about how the dedicated venous open-cell nitinol Abre stent (Medtronic) compares with other venous nitinol stents available, noting that it is very easy to use,...
Writing for Venous News, Paul Gagne considers the future of intravascular ultrasound (IVUS) based therapies for venous leg ulcers (VLUs). He concludes that while studies of IVUS guided stenting to date "have moved the ball far down the field,"...
The Charing Cross (CX) Symposium, with its world-class faculty and unique focus on live audience participation, has announced the launch of a not-to-be missed and timely vascular and endovascular education virtual event: CX 2020 LIVE.
Mark your calendars for this...
Compression stockings might be unnecessary to prevent blood clots in most patients undergoing non-emergency (elective) surgery, finds a clinical trial published by The BMJ today.
The results of the Graduated compression stockings as adjuvant to pharmaco-thromboprophylaxis in elective surgical patients...
Control Medical Technology has announced that the US Food and Drug Administration (FDA) has cleared its Aspire MAX 7 – 11F mechanical thrombectomy platform to remove blood clots from peripheral vessels.
"This FDA clearance quadruples our product offering and...
Manj Gohel (Cambridge, UK), one of the editors-in-chief of Venous News, introduces a special COVID-19 video with leading thrombosis and haemostasis experts, including Beverley Hunt (London, UK), Ian Franklin (London, UK), Francis Matthey (London, UK) and Sriram Narayanan (Singapore),...
The venous space continues to evolve in the United States, and many physicians are learning how to navigate through the challenges of the venous anatomy from their international peers. As a well-respected expert, the Galway-based interventional radiologist Dr. Gerard...
Venous News talks with Gerard O'Sullivan, FSIR, FEBIR, University College Hospital, Galway, Ireland. This article is supported by BD Interventional.
Disclosures: Dr. Gerard O'Sullivan reports he is a consultant/speaker for BD Interventional, Aspirex, Boston Scientific Corporation, Cook Medical, Marvao Medical,...
Prism Schneider (Cumming School of Medicine, University of Calgary, Calgary, Canada) and others write in a commentary in the Canadian Medical Association Journal—because of an increase in domestic violence during the pandemic—healthcare providers should be aware of the signs...
There is an “urgent need” to improve specific venous thromboembolism (VTE) diagnostic strategies and investigate the efficacy and safety of thromboprophylaxis in ambulatory COVID-19 patients. This is the conclusion of a recent study into venous and arterial thromboembolic complications...
A 2020 update on the Clinical-Etiology-Anatomy-Pathophysiology (CEAP) classification system and reporting standards, authored by Fedor Lurie (Jobst Vascular Institute, Toledo, USA; University of Michigan, Ann Arbor, USA) and colleagues, has been published in the Journal Vascular Surgery: Venous and...
On 18 March, the Centers for Medicare & Medicaid Services (CMS) recommended “limiting non-essential care and expanding surge capacity into ambulatory surgical centres and other areas” to conserve resources and staff for managing COVID-19 patients. However, in a statement...
The COVID-19 subcommittee of the American Venous Forum (AVF) has issued a white paper on considerations in prophylaxis and treatment of venous thromboembolism (VTE) in COVID-19 patients.
Jeffrey Mendola, director of Mission Advancement at the AVF, emphasises the importance...
Joseph A Caprini considers venous thromboemolism (VTE), patient risk assessment, and therapeutic challenges in the wake of the COVID-19 pandemic, concluding that "accurate thrombosis risk assessment for all coronavirus patients is advised".
Emergence of the coronavirus worldwide represents a pandemic...
In a brief report in Annals of Internal Medicine, Seongman Bae (Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea) and colleagues report that neither surgical nor cotton masks appear to be an effective approach for preventing the dissemination of SARS-CoV-2 from...
Manj Gohel, Stephen Black and Alun Davies consider the impact of the COVID-19 pandemic on patients with venous disease. They conclude: "Once the acute crisis has passed, we must work hard as a community to avoid the population of...
Royal Philips has announced that the US government and Philips agreed to team up to increase the production of hospital ventilators in its manufacturing sites in the USA.
Philips plans to double the production by May 2020 and achieve...
The European Commission (EC) has adopted a proposal to postpone by one year the date of application of the new Medical Devices Regulation (MDR), which was due to come into force on 26 May this year. The postponement, a press...
In a new statement published on 2 April 2020, all 45 societies represented by the US Council of Medical Specialty Societies (CMSS)—over 800,000 physicians—emphatically declare their belief that all frontline healthcare professionals must have access to personal protective equipment...
The American College of Surgeons (ACS) today leapt to the defense of healthcare workers who are heading to work in the face of increasing concerns over personal protective equipment (PPE) shortages as the COVID-19 pandemic cuts trails across the...
As a podium talk at the annual meeting of the American Venous Forum (VENOUS 2020; 3–6 March, Amelia Island, USA), it represented a bit of an outlier. It was, the presenter admitted, the only non-scientific talk on the programme....
Mechanochemical ablation (MOCA) is a superior option to endovenous laser ablation (EVLA) for the treatment of primary varicose veins, according to the findings of a randomised controlled trial which compared the two modalities. Published in the Journal of Vascular...
It has been found that oral apixaban is noninferior to subcutaneous dalteparin for the treatment of cancer-related venous thromboembolism (VTE), as revealed by Giancarlo Agnelli (University of Perugia, Perugia, Italy) at the American College of Cardiology/World Congress of Cardiology’s...
Results of PRONOMOS, a randomised, controlled, noninferiority trial, have shown rivaroxaban to be more effective than enoxaparin in the prevention of major venous thromboembolic events, during a period of immobilisation after non-major orthopaedic surgery of the lower limbs. The...
During a Q&A section of a European Commission (EC) college meeting on 25 March, EC spokesperson Stefan de Keersmaecker stated that the commission were looking to delay the “entry into force” of the new European medical device regulations (MDR) because of...
A viewpoint in the Journal of the American Medical Association (JAMA) has offered potential solutions to modifying ongoing randomised clinical trials during the COVID-19 pandemic. It aims to “minimise disruption and preserve integrity”, while still ensuring participant health and safety.
Authors Mary...
Use of autologous skin cell suspension (ASCS) in addition to compression has been shown to accelerate healing in large venous leg ulcers (VLUs), according to the results of a multicentre randomised trial conducted in the UK. Writing in the...
The American College of Surgeons (ACS) has announced the release of new clinical guidance for surgeons to curtail recommendations for elective surgical procedures, with the aim of preserving necessary resources for the care of critically ill patients during the...
The 2020 Charing Cross (CX) Symposium that was due to take place next month in London, UK, has been cancelled due to COVID-19.
In full, the statement from the CX Symposium team, reads:
"The CX Symposium team has made the difficult...
The US Food and Drug Administration’s (FDA) Center for Devices and Radiological Health (CDRH) has written to the medical device industry to outline its response to the COVID-19 public health emergency in its day-to-day operations with industry.
The letter from...
Incorporating evidence collected since 2004, the latest revision of the CEAP (Clinical-Etiology-Anatomy-Pathophysiology) classification system has underlined a number of key updates relating to descriptions of chronic venous disease patients. “There has been a lot of information that has come...
“We found deficiencies in current indications for intervention, which may explain some of the overutilisation we have been encountering,” explained Elna Masuda (Straub Medical Center, Hawaii Pacific Health, Honolulu, USA), during an overview of the new 2020 appropriate use...
The Lindsay Leg Club Foundation has launched a new fundraising campaign in partnership with the Aviva Community Fund, in a bid to raise money for a new Leg Club. There are currently 44 Leg Clubs operating throughout the UK,...
According to a network meta-analysis, the VenaSeal system (Medtronic) is “a promising therapeutic option for anatomic success at six months”. This study, published in the Journal of Vascular Surgery: Venous and Lymphatic Disorders, also highlighted the lower number of...
Results of a 12-month study conducted by a group of Commonwealth physicians have demonstrated that endovenous valve formation in the deep venous system is feasible. Presented in the International Session at the annual meeting of the American Venous Forum...
Symptoms of restless leg syndrome are 34.5% more prevalent in patients with superficial venous insufficiency, according to a retrospective study presented by Aaron Dezube (St Elizabeth Medical Center, Brighton, USA). Although restless leg syndrome was identified in those without...
Thrombolex has announced that results of its First-In-Human (FIH) trial confirm the early safety and feasibility of using the Bashir Endovascular Catheter for pharmacomechanical catheter-directed thrombolysis (CDT), in patients with acute pulmonary embolism (PE).
The data, presented at the...
“Venous leg ulcers (VLUs) secondary to deep venous stenosis represents a distinct class of patients who require a unique treatment paradigm,” stated Abhisekh Mohapatra (University of Pittsburgh Medical Center, Pittsburgh, USA), presenting results of a multicentre retrospective study of...
A retrospective review of patients presenting with bilateral obstructive iliofemoral venous lesions has shown that contralateral limb symptoms improve following stenting of the worse ipsilateral limb. In addition to this, the study found that only a small proportion of...
In a case-control study of patients with perceived contraindications to anticoagulation, who either received or did not receive inferior vena cava (IVC) filters, no difference was found in the incidence of symptomatic pulmonary embolism (PE) at 90 days.
Presented...
Access Vascular has announced that it has received clearance from the US Food and Drug Administration (FDA) for the second generation of its HydroPICC peripherally inserted central catheter (PICC), which has demonstrated a thrombus accumulation rate 30 times less...
MolecuLight has announced the release of a "significant upgrade" to its I :X digital wound measurement feature that enables clinicians to more accurately and quickly capture digital wound area measurement. According to a statement, clinicians can now reliably detect more...
ATTRACT compared outcomes following pharmacomechanical thrombolysis (PMT) versus standard medical therapy (SMT), and while no benefit with PMT was demonstrated for femoropopliteal deep vein thrombosis (DVT), subgroup analysis of iliofemoral DVT demonstrated a reduction in moderate-to-severe post thrombotic syndrome...
Use of the ClotTreiver system (Inari Medical) for the treatment of lower extremity deep vein thrombosis (DVT) has demonstrated safety and efficacy, especially in “removing large volumes of lower extremity acute thrombus in a single session”. A retrospective review...
Investigators in The Netherlands, France and Belgium have developed a risk assessment model for venous thromboembolism (VTE). Published by The Lancet in EClinicalMedicine, the TRiP(cast) score (thrombosis risk prediction following cast immobilisation) is described as “a helpful tool in...
A retrospective analysis of patients diagnosed with lower extremity lymphoedema, and treated in a cancer-affiliated physical therapy department, has found that chronic venous insufficiency (or phlebolymphoedema) is the “predominant cause of lower extremity lymphoedema”. Published online in the Journal...
Following the publication of “Another nail in the coffin of vena cava filters” in the British Journal of Surgery, which considered potential problems with the use of inferior vena cava (IVC) filters, authors Andrew Doyle and Narayan Karunanithy (Guy’s...
Bluegrass Vascular Technologies (Bluegrass Vascular) announced today that the US Food and Drug Administration (FDA) has granted a de novo classification order for its Surfacer Inside-Out access catheter system. The Surfacer system is intended to obtain central venous access...
XableCath has announced that its XableCath Crossing catheters have received CE mark for peripheral use. Its crossing devices will be available for sale in Europe in two versions, a blunt tip and an abrasion tip, in a variety of...
Venous News filmed a panel discussion with Alun Davies (London, UK), Manj Gohel (Cambridge, UK) and Daniel Carradice (Hull, UK); who discussed treatment of venous ulceration and the randomised EVRA trial which, Carradice says, showed that early endovenous ablation of superficial venous...
“We need to check for signs of recurrence and functional impairment, and follow these patients much more closely,” urged Kenneth Rosenfield (Massachusetts General Hospital, Boston, USA), speaking on the long-term outcomes for pulmonary embolism (PE) patients at the International...
Michael Lichtenberg (Arnsberg, Germany) talks to BLearning Venous at LINC 2020 (Leipzig Interventional Course; 28–31 January 2020, Leipzig, Germany) about the rationale behind his team’s study which looked at Intravascular ultrasound (IVUS) compared with fluoroscopic-guided recanalisation in patients with...
According to the findings of a European multicentre retrospective analysis, the use of drug-coated balloons (DCBs) for the treatment of symptomatic central venous stenosis in dialysis patients has been demonstrated as safe. Presented at the Leipzig Interventional Course 2020...
“When we are talking about varicose veins, we are talking about patients, all of whom have different patterns of varicose veins,” stated Armando Mansilha (University of Porto, Porto, Portugal), responding to the argument of Thomas Proebstle (University of Mainz,...
Clinical outcomes with the Vici venous stent system (Boston Scientific) have been described as “very good”, according to a presentation of 24- and 36-month follow-up data at the Leipzig Interventional Course 2020 (LINC; 28–31 January, Leipzig, Germany). During his...
Biolitec has announced the addition of another laser fibre to its ELVeS Radial 2ring laser fibre range. The ELVeS Radial 2ring Pro enables the minimally invasive endovenous laser treatment of severely tortuous veins.
According to a press release, the ELVeS...
Raghu Kolluri (Columbus, USA) and John Kaufman (Portland, USA) talk to BLearning Venous about the highlights from The VEINS (Venous Endovascular INterventional Strategies) session at VIVA 2019 (Vascular InterVentional Advances; 4–7 November, Las Vegas, USA), as well as the...
Initial results of an ongoing first-in-man study in Colombia, that saw the implantation of a prosthetic venous valve in 15 patients, have demonstrated an improvement in clinical outcomes and quality of life. The promising findings were presented to delegates...
Sky Medical Technology has announced that the geko device was presented as part of the didactic program, and at a corporate-sponsored symposium, at the Orthopaedic Summit 2019: Evolving Techniques (OSET 2019) annual meeting (December 11–14, Las Vegas, USA).
Jason Snibbe, a...
A retrospective analysis of patients treated with ultrasound-guided foam sclerotherapy (UGFS), published in the January 2020 issue of Phlebology, has found that the frequency of non-target vein occlusions following this procedure—revealed by serial duplex ultrasound (DUS)—could be as high...
Argon Medical Devices has announced the commercial launch of its Single-loop and Triple-loop retrieval kits for sale in the USA. The Single-loop and Triple-loop Retrieval Kits are intended for percutaneous removal of retrievable inferior vena cava (IVC) filters that...
Following his recent appointment as president of the Society for Vascular Medicine (SVM), Raghu Kolluri (Columbus, USA) speaks to Venous News about how the venous field is being affected by the results of recent trials, his involvement with The...
The “Standard versus ultrasound-assisted catheter thrombolysis for submassive pulmonary embolism” (SUNSET sPE) trial, a randomised, single-blinded clinical trial comparing ultrasound-assisted thrombolysis (USAT) to standard catheter-directed thrombolysis (SCDT), has completed enrolment, as announced on Friday 17 January by principal investigators...
In this issue of Venous News:
CAVA trial: CDT does not change post-thrombotic risk for iliofemoral DVT patients
Restless legs study establishes association with reflux of lateral subdermic plexus
Manj Gohel and Stephen Black: Where does the CAVA trial leave us?
...
In this issue of Venous News:
CAVA trial: CDT does not change post-thrombotic risk for iliofemoral DVT patients
Restless legs study establishes association with reflux of lateral subdermic plexus
Manj Gohel and Stephen Black: Where does the CAVA trial leave us?
...
Penumbra has announced US Food and Drug Administration (FDA) 510(k) clearance for expanded indication of the Indigo Aspiration System. As part of the system, Indigo Aspiration Catheters and Separators are indicated for the removal of fresh, soft emboli and...
With the level of spending on wound care in the billions of pounds, both the financial and health-related impact of venous leg ulcers cannot be understated. Dan Carradice (Hull York Medical School, Hull, UK) speaks to Venous News about...
Results from the Dutch CAVA trial were published recently in The Lancet Haematology and, at first glance, this is another trial that failed to demonstrate any meaningful benefit for early thrombus removal in patients with acute iliofemoral deep vein...
Serena Auñón-Chancellor, clinical associate professor of medicine at Louisiana State University Health, New Orleans School of Medicine’s branch campus, in Baton Rouge, USA, is the lead author of a paper which describes a previously unrecognised risk of spaceflight discovered...
An analysis of data from the ATTRACT trial, published in the Journal of Vascular Surgery: Venous and Lymphatic Disorders (JVSVL), reveals that quality of life measures improve after pharmacomechanical catheter-directed thrombolysis (PCDT). According to the findings of the analysis,...
Insera Therapeutics’ CLEAR aspiration system has been used for the first time to treat a (human) patient with acute pulmonary embolism (PE). CLEAR, “cyclical luminal, evaluation, aspiration, and retrieval”, is a digital vacuum aspiration system that allows operators to...
The US Food and Drug Administration (FDA) has announced the approval of two applications for the first generics of Eliquis (apixaban) tablets, to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.
Apixaban is also...
Bluegrass Vascular Technologies, a private medical technology company focused on innovating devices and methods for vascular access procedures, has announced the publication of positive results associated with a clinical study involving the use of the Surfacer Inside-Out Access Catheter...
“The goal needs to remain on improving healing, but also reduced recurrence in the long term,” said Stephen Black (Guy’s and St Thomas’ Hospital, London, UK), during his presentation of results from the Leg Ulcer Pathway Audit (LUPA) study...
Narayan Karunanithy outlines the benefits of intravascular ultrasound (IVUS) during haemodialysis access intervention.
Thoracic central veins include intra-thoracic segments of the internal jugular, subclavian, brachiocephalic veins and superior vena cava. These veins are located central to the superior thoracic aperture...
Thrombolex has announced that the company has received US Food and Drug Administration (FDA) clearance for the Bashir Endovascular Catheter - Short Basket (BEC S-B). The indication for use of the BEC S-B is for the controlled and selective...
While the treatment of spider, reticular and varicose veins using sclerotherapy has been a standard practice for many years, tumescent enhanced sclerotherapy has been developed as an office-based technique that takes traditional sclerotherapy a step further by applying a...
Results of a randomised controlled trial conducted at Imperial College, London, UK, have shown that the application of compression stockings is associated with a significant reduction in pain during the first few days following intervention. In addition, it was...
Based on the results of a retrospective, single-centre cohort study conducted at The RANE Center, St Dominic’s Memorial Hospital (Jackson, Mississippi, USA), the importance of intravascular ultrasound (IVUS) for identifying the location and severity of stenotic lesions in the...
Insera Therapeutics announced recently that the first patient with acute pulmonary embolism has been treated with its flagship cyclical aspiration system, CLEAR. Earlier last month, the first two stroke patients were treated using the same platform. The company received...
Additional ultrasound-accelerated catheter-directed thrombolysis (CDT) does not change the risk of post-thrombotic syndrome in patients with acute iliofemoral deep vein thrombosis (DVT) at one-year follow up, compared with standard therapy alone, according to findings of the CAVA trial published...
There are “significant limitations” to the use of duplex ultrasound for the initial evaluation of patients with suspected subclavian vein (SCV) thrombosis, according to the findings of a retrospective, single-centre review published in the Journal of Vascular Surgery: Venous...
Writing to members of the American Venous Forum (AVF), president Brajesh K Lal (Baltimore, USA), has revealed that the AVF has established a partnership with the C-TRACT study, a randomised controlled trial funded by the National Institutes of Health....
Long-term evaluation of superficial venous reflux below the knee, in patients who received invasive treatment for varicose veins, has found that both radiofrequency ablation (RFA) and open surgery achieve "acceptable and comparable results" in the elimination of reflux in...
In the treatment of reticular veins of the lower extremities, using polidocanol foam, it has been found that both a 1:2 ratio and 1:4 ratio of polidocanol to air demonstrate similar levels of efficacy and safety. According to presenter...
Findings of a recent study on whether a pathologic perforator can predict the presence of an ipsilateral central venous stenosis, as presented at the annual meeting of the American Vein and Lymphatic Society (AVLS; 7–10 November 2019, Arizona, USA),...
Implementation of an evidence-based pathway for leg ulcer care in community nursing has resulted in a significant reduction in healing times, according to research conducted by the Manchester University NHS Foundation Trust, Manchester, UK. Although quality of life scores...
As course director of The VEINS at VIVA 2019 (2–4 November, Las Vegas, USA), John Kaufman tells Venous News how the field of interventional radiology (IR) has transformed over the years and highlights key areas of innovation to look...
Miles D White will be stepping down, after 21 years, as CEO of Abbott on 31 March 2020. According to a press release, White’s tenure is the second longest for a non-founder in today’s S&P 100; he will remain...
Reflux of the lateral subdermic plexus—varicose veins which appear on the outside of the thigh and calf—could be closely related to the symptoms of restless leg syndrome and night-time cramping, according to a review conducted on prospective symptom tracking...
Clinically relevant outcomes are needed to measure catheter-directed therapy (CDT) against anticoagulation alone to treat submassive pulmonary embolism (PE) patients. Further, CDT’s ability to affect short-term and long-term outcomes such as cardiopulmonary health after PE needs “better characterisation”, Akhilesh...
David J. Dexter, (Sentara Healthcare, Norfolk, VA, USA), principal investigator of the CLOUT registry using the ClotTriever Mechanical Thrombectomy System (Inari Medical) for treatment of acute and chronic lower extremity DVT, presented the initial results for the first 50...
Suresh Vedantham (St Louis, USA) talks to BLearning Venous after winning the prestigious VEINS award at VIVA 2019 (Vascular InterVentional Advances; 4–7 November, Las Vegas, USA) about his current take on the open vein hypothesis and more.
Vedantham reflects on...
In this issue of Venous News:
Future trials on DVT management "have to focus on technical success"
Foam "has not been established as superior" to liquid sclerotherapy
Manj Gohel: A look at new preliminary guidelines for SVT
Anelise Rodrigues: Augmented reality...
In this issue of Venous News:
Future trials on DVT management "have to focus on technical success"
Foam "has not been established as superior" to liquid sclerotherapy
Manj Gohel: A look at new preliminary guidelines for SVT
Anelise Rodrigues: Augmented reality...
Inari Medical has announced the presentation of early outcomes from the ClotTriever Outcomes Registry (CLOUT) using the ClotTriever Mechanical Thrombectomy System for treatment of acute and chronic lower extremity deep vein thrombosis (DVT).
Initial results for the first 50...
It has been found by the EXTRACT-PE trial, a multicentre investigation conducted under an investigational device exemption (IDE) from the US Food and Drug Administration (FDA), that aspiration thrombectomy for acute pulmonary embolism (PE) with the Indigo Aspiration System...
A Philadelphia jury has awarded more than US$33 million to a woman who was injured as a result of a defectively designed inferior vena cava (IVC) filter. The trial was overseen by Philadelphia Judge Michael Erdos.
The litigation focused on...
Analysing the results of a retrospective, single-centre cohort study conducted at Guys and St Thomas’ Hospital, London, UK, Anna Pouncey (Guys and St Thomas’ Hospital, London, UK) emphasised that over 50% of cases included in the investigation could have...
Speaking at the annual meeting of the Cardiovascular and Interventional Radiological Society of Europe (CIRSE 2019; 7–11 September, Barcelona, Spain), Stephen Black (Guys and St Thomas’ Hospital, London, UK) emphasised that “ATTRACT and CAVA are not the end of...
It has been found that the application of dehydrated human amnion and chorion allografts (dHACA) results in a better venous leg ulcer (VLU) healing rate than the use of standard multilayer compression therapy, following a randomised controlled study comparing...
Current classification tools for the diagnosis of chronic venous disease, including acute deep vein thrombosis (DVT) and post-thrombotic syndrome (PTS), include “redundant” elements that are unimportant to patients, according to an address delivered by Mark Meissner (Seattle, USA) at...
Venarum Medical has announced that it has received a mentorship grant in further support of its awarded Phase II SBIR Grant “EVVS: EndoVenous Valve System”, which is currently underway.
Under its Phase II work, Venarum proposes to verify the preclinical...
A report titled ‘Venous Leg Ulcers: A Silent Crisis’ has been published by the All-Party Parliamentary Group on Vascular and Venous Disease (VVAPPG) at an event held at the Palace of Westminster, highlighting the opportunities available to help thousands...
At the European Society of Vascular Surgery annual meeting (ESVS 2019; 24-27 September, Hamburg, Germany), preliminary venous thrombosis guidelines were presented for the first time. Chaired by Stavros Kakkos (Patras, Greece) and Manj Gohel (Cambridge University Hospitals, Cambridge, UK),...
Indocyanine green dye (ICG) fluorescence lymphography represents a suitable method of providing information on the effectiveness of intermittent pneumatic compression (IPC) during the treatment of leg oedema (an excess of fluid around the limb), according to a study conducted...
STARmed has announced that it has obtained CE Mark approval for VENISTAR, an "innovatively designed" electrode that uses radiofrequency energy to treat patients with damaged veins. According to a statement, this minimally invasive electrode provides physicians with the ability...
PolarityTE, a company specialised in regenerative tissue products and biomaterials, has announced findings from an open-label, single-arm pilot study, which examined the impact of SkinTE in closing venous stasis leg ulcers (VLUs) following the failure of conventional treatments.
The clinical outcomes...
A pilot evaluation of a new multilayer wound dressing has confirmed its efficacy for transporting exudate containing toxic matrix metalloproteases (MMPs) away from chronic venous leg ulcers (VLUs). Conducted by Oscar M Alvarez (University Hospital; Rutgers New Jersey Medical...
UK-based Sky Medical Technology has received US Food and Drug Administration (FDA) 510(k) clearance for its "geko" device for stimulation of the calf muscles to prevent venous thrombosis in non-surgical patients at risk for venous thromboembolism (VTE).
This adds...
Speaking at the International Union of Phlebology chapter meeting (UIP 2019; 25–27 August, Krakow, Poland), Neil Khilnani (New York, USA) underlined that the current literature on sclerotherapy, in addition to guidelines from the European Consensus and American Society for...
Kurosh Parsi, incoming president of the International Union of Phlebology, or Union Internationale de Phlebologie (UIP), is an Australian dermatologist, phlebologist and vascular anomalies specialist who pioneered catheter-guided and tumescent-assisted sclerotherapy.
With his broad range of expertise and varied...
Clinical data presented at the 2019 European Cardio-Thoracic Surgery (EACTS) annual meeting (3-5 October, Lisbon, Portugal), from two prospective trials of the VEST external stent for vein grafts during coronary artery bypass surgery (CABG), showed low major adverse cardiac...
At the 2019 Transcatheter Cardiovascular Therapeutics (TCT) meeting (25–29 September, San Francisco, USA), InterVene won the TCT-Shark Tank Innovation Competition for its novel endovenous system (BlueLeaf) for forming valves in patients with deep vein reflux and/or chronic venous insufficiency. The award, which...
In this issue of Venous News:
SUPER trial demonstrates benefits of intermittent pneumatic compression
"Fascinating vision" of non-invasive varicose vein treatment
Oliver Lyons: Pharmacotherapy for lymphoedema
Efthymios Avgerinos: SUNSET sPE interim outcomes
Profile: Kurosh Parsi
In this issue of Venous News:
SUPER trial demonstrates benefits of intermittent pneumatic compression
"Fascinating vision" of non-invasive varicose vein treatment
Oliver Lyons: Pharmacotherapy for lymphoedema
Efthymios Avgerinos: SUNSET sPE interim outcomes
Profile: Kurosh Parsi
Like many other industries, the shape of healthcare and medicine is being transformed by the emergence of new technologies. Phlebology, as Anelise Rodrigues (Culabá, Brazil) explains, is no different. While there are still concerns with the potential limitations and...
Laser crossectomy of the GSV has been established as a more effective method of preventing secondary anterior accessory great saphenous vein (AAGSV) reflux than treatment modalities which leave stumps. As part of a randomised controlled study comparing the technique...
Bluegrass Vascular Technologies (Bluegrass Vascular) has announced the publication of positive results associated with the clinical use of the Surfacer Inside-Out access catheter system in the peer-reviewed Journal of Vascular Access (JVA).
Results of an independent, single-centre study demonstrated successful use...
The Janssen Pharmaceutical Companies of Johnson & Johnson has announced the publication of new real-world evidence which confirms that XARELTO (rivaroxaban) reduces the risk of recurrent venous thromboembolism (VTE) in patients who are morbidly obese, with effectiveness and safety...
Sanjay Misra (Rochester, USA) talks to Interventional News about his research into whether stem cell therapy can help AVFs (arteriovenous fistulas) mature and also prevent venous stenosis. The pilot study with a small group of haemodialysis AVF patients led to “promising...
While compression therapy is of the treatment options for patients suffering with venous insufficiency and chronic wounds, this could also present benefits for healthy subjects. Following his presentation at the International Union of Phlebology chapter meeting (UIP 2019; 25–27...
There is no significant difference in pain and recanalisation for patients undergoing endovenous laser ablation (EVLA) at different power settings, according to the results of a new randomised controlled clinical trial. This investigation was conducted following a previous morphological...
Evidence from a recent study conducted by Imperial College London, UK has further demonstrated that the Villalta scoring system is not disease-specific and correlates with other tools for measuring venous outcomes. The results of the study have implications for...
PIUR Imaging has launched a portable version of its tomographic ultrasound system to the European market. The new CE-marked PIUR tUS Infinity is a small, wireless version of its tomographic ultrasound solution that turns any standard ultrasound into a...
Omar Ishrak, Medtronic’s chairman and CEO is to retire on 26 April 2020, following the end of the company’s current fiscal year. Also, the Medtronic Board of Directors has announced key leadership appointments as part of its multi-year, leadership...
Michael Dake (Tucson, Arizona) presented the two-year data from the VERNACULAR trial at CIRSE 2019 on 8 September in Barcelona, Spain.
Dake said: “The 24-month results from the VERNACULAR trial using the BD Venovo stent show absolutely sustained benefit in...
Boehringer Ingelheim has announced the publication of primary analysis from RE-SPECT CVT, the first exploratory, prospective, randomised controlled study of a NOAC in patients with blood clots in the veins or venous sinuses of the brain. The trial investigated...
An updated guideline on the prophylaxis and treatment of venous thromboembolism (VTE) in patients with cancer has been unveiled by the American Society of Clinical Oncology (ASCO) in its Journal of Clinical Oncology. An expert panel convened by ASCO...
A study into the efficacy of early reflux elimination for venous leg ulcer (VLU) patients has revealed that the combination of ultrasound-guided foam sclerotherapy (UGFS) only, and a hybrid method of endovenous laser treatment (EVLT) with UGFS, could present...
A private equity firm, Palatine (Manchester, UK), has announced that it has made a “significant investment” into a UK centre—Veincentre—that provides endovenous laser ablation (EVLA) for the management of varicose veins. The investment, according to Palatine, is the fourth...
Results from two open prospective trials have revealed that TLC-NOSF sucrose octasulphate dressing, with poly-absorbent fibres, represents an effective and safe treatment for the local management of leg ulcers. In addition, cost-effectiveness studies examining the economic impact of the...
While developing specific treatment for primary lymphoedema presents an “enormous” scientific challenge, secondary lymphoedema may prove an easier target, Oliver Lyons writes for Venous News. In this article, Lyons outlines what is currently known, and what to expect in...
Certain reflux patterns in patients with chronic venous disease can be associated with increased clinical severity and a lower quality of life, according to the results of a recent study conducted at Imperial College London, UK. As part of...
Thrombolex has announced the enrolment of the first patient in their Early Feasibility and Safety Study, investigating the Bashir Endovascular Catheter for the treatment of submassive pulmonary embolism (PE). The device was designed to quickly and safely dissolve thrombus...
A member of the UK parliament has emphasised the need for a national wound care strategy during a recent debate in the House of Commons, highlighting that “diagnosis is difficult, but rapid diagnosis is absolutely essential”. Ann Clywd, MP...
Karen Bauer (Toledo, USA) discusses the wound care limb salvage track at C3 which offered the “opportunity for clinicians from multiple disciplines to get together and talk about some really cutting-edge” topics.
Bauer, a wound specialist, says that at the...
In her presentation at the Royal Society of Medicine Venous Forum (RSMVF; 17–19 June, London, UK), consultant vascular surgeon Emma Wilton (Oxford University Hospitals, Oxford, UK) outlined the important process for identifying which patients with leg ulceration require deep...
Endovenous laser ablation (EVLA) of perforating veins has been further established as an effective method of treating chronic venous ulcers in patients with diabetes mellitus, according to a new study conducted in Azerbaijan.
Results of the study, which involved 42...
A recent study has concluded that patients with inherited or acquired thrombophilia should not be excluded from ilio-femoral venous stenting, Adam Gwozdz (St Thomas’ Hospital, London, UK) reported at the European Venous Forum (EVF; 27–29 June, Zurich, Switzerland). “By...
The Janssen Pharmaceutical Companies of Johnson & Johnson announced today new results from the Phase 3 EINSTEIN-Jr study, showing paediatric patients (aged birth to 17 years) treated with rivaroxaban (brand name Xarelto) had a similar low risk of recurrent...
A US-based multicentre study has found that while chronic venous insufficiency is “primarily observed in white women”, there are other notable differences in the incidence and prevalence of disease severity and outcomes when comparing patients’ racial groups, with African...
Rabih Chaer (Pittsburgh, USA) talks to Venous News at the Society for Vascular Surgery’s Vascular Annual Meeting (SVS VAM; 12–15 June 2019, Washington, DC, USA)about the “need for dedicated venous stents” in the US in anticipation of the recently US FDA approved...
InterVene has announced it has raised US$15 million in a Series B financing round. The company’s BlueLeaf Endovenous Valve Formation System is the first catheter-based solution for deep venous reflux that does not require an implant.
The system is designed to correct one...
The new West London Vascular and Interventional Centre at Northwick Park Hospital (Harrow, UK) has officially been declared open. As of the 25th June, 2019, patients who require vascular treatment will be able to benefit from the modern facilities...
Siemens Healthineers and Mentice AB have announced that they are collaborating to fully integrate Mentice’s VIST virtual patient into the Artis icono angiography system from Siemens Healthineers.
According to a press release, the VIST virtual patient thus becomes a fully...
Catheter-directed thrombolysis improves outcomes and long-term cost efficiency for treatment of massive and submassive pulmonary embolism (PE), in comparison to systemic heparin administration. This was the conclusion of study investigators led by Patrick Kelly (Sanford Health, Sioux Falls, USA),...
The Standard vs ultrasound-assisted catheter thrombolysis for submassive pulmonary embolism (SUNSET sPE) trial, is an ongoing randomised, head-to-head, single-blinded clinical trial comparing ultrasound-assisted thrombolysis to standard catheter-directed thrombolysis. While comparative data is not yet available, Efthymios "Makis" Avgerinos (University...
In this issue:
- "Urgent action" is required to improve referral for venous ulcer patients
- VenaSeal maintains safety and efficacy at extended five-year follow-up
- Dominik Heim: The 20th European Venous Forum (EVF)
- Raghu Kolluri: The role of elevated central venous...
In this issue:
- "Urgent action" is required to improve referral for venous ulcer patients
- VenaSeal maintains safety and efficacy at extended five-year follow-up
- Dominik Heim: The 20th European Venous Forum (EVF)
- Raghu Kolluri: The role of elevated central venous...
The TLC-NOSF (UrgoStart, Urgo Medical) advanced wound care dressing was recently recommended by the UK National Institute for Health and Care Excellence (NICE) for healing of venous and diabetic foot ulcers. Speaking at the European Wound Management Association annual...
Fedor Lurie started his medical career in Ekaterinburg, Russia, on the east side of the Ural mountain chain. Now a long-time prominent figure in some of the largest venous and vascular societies in the USA, Lurie speaks to Venous...
Steven Dubenec (Sydney, Australia) and Gerard O’Sullivan (Galway, Ireland) discuss the impact of dedicated venous stents, in particular the Venovo venous stent (BD, formerly Bard) at CX 2019.
Dubenec talks about how the Venovo stent – which comes in a...
The last two decades have seen a major revolution in the treatment of varicose veins and venous reflux disease—and we may be on the brink of the next big shift. This is what Mark S Whiteley writes for Venous...
Michael Edmonds (London, UK), Thomas Serena (Warren, USA), Keith Harding (Cardiff, UK), and Williams Ennis (Oak Lawn, USA) talk at CX Live about the first ever iWounds workshop, the importance of wound management and what they hope to achieve.
Edmonds...
A new study has found that intensive care unit patients with swine flu (H1N1) and acute respiratory distress syndrome (ARDS) were 33 times more likely to develop venous thromboembolism (VTE), unless prescribed with an anticoagulant when admitted.
The findings were...
Vesper Medical, a developer of medical devices for minimally invasive peripheral vascular procedures, has announced a US$37 million financing with Vensana Capital and Gilde Healthcare as lead investors. They were joined by existing investors New Enterprise Associates (NEA) and...
In this article for Venous News, Dominik Heim spoke to Michel Perrin and Bo Eklöf about the founding of the European Venous Forum (EVF; 27–29 June, Zürich, Switzerland), an international symposium for venous specialists that this year celebrated its...
Defining success in venous ulcer healing, and for all chronic wounds, remains a nebulous concept—with no consensus on what constitutes a successful outcome. Dave Bosanquet (Bristol, UK) outlined the difficulties at the Charing Cross Symposium (CX; 15–18 April, London,...
A new treatment modality is aiming to take the idea of minimally-invasive procedures to the next level for treatment of varicose veins and venous insufficiency. Alfred M Obermayer of the Institute of Functional Phlebosurgery, Karl Landsteiner Society in Vienna,...
Thrombolex has announced that the US Food and Drug Administration (FDA) has cleared the Bashir Endovascular Catheter (BEC) for the controlled and selective infusion of physician-specified fluids, including thrombolytics, into the peripheral vasculature, and the Bashir N-X Endovascular Catheter...
Inari Medical has announced the publication of its 106-patient prospective multicentre FlowTriever Mechanical Pulmonary Embolectomy (FLARE) study for the treatment of intermediate-risk pulmonary embolism (PE). The study was published in JACC: Cardiovascular Interventions. FLARE was conducted under the direction...
Boston Scientific has announced hat the US Food and Drug Administration (FDA) has approved the Vici venous stent system for the treatment of iliofemoral venous obstructive disease.
Venous obstructive disease affects nearly 40% of the population in the USA and...
Also known as "Vein Specialists Sitting around Talking and Having Drinks," the VEIN Magazine Podcast with Steve Elias features real discussions on venous disease and treatment. Once a month, this podcast brings together leaders in the venous treatment world...
Prakash Saha (London, UK), speaking during the Venous and Lymphatic Challenges programme at CX (15–18 April, London, UK), outlined the three key steps to optimising treatment of acute deep vein thrombosis: identification, management, and surveillance. He added that the...
Raghu Kolluri gives an overview of elevated central venous pressure—a condition which Kolluri argues is elusive, underdiagnosed and in need of further examination in order to establish its role and relationship to chronic venous insufficiency.
It is not uncommon to...
“A lot more bacteria than anticipated” have been discovered on wounds in a patient cohort consisting of 89% venous leg ulcers, and using fluorescence imaging proved superior for detecting infection in wounds. This was the conclusion of Tomas Serena (Warren,...
Despite what current guidelines denote, and in addition to the emergence of data supporting the early intervention of venous ulceration, Alun Davies (London, UK) put forward that the recommendations are consistently being ignored, and highlighted that: “Urgent action is...
The first-ever 60-month data on Medtronic’s VenaSeal closure system were presented on Tuesday, indicating that at five years, treatment with the cyanoacrylate adhesive for closure of diseased vein segments was not inferior to the alternative treatment arm of radiofrequency...
Speaking at the Charing Cross International Symposium (CX; 15–18 April, London, UK), Isaac Nyamekye (Worcester, UK) presented the results of the 3RF study, which compared three radiofrequency ablation systems (Venefit, RFITT, and EVRF). Nyamekye reported that compared with the...
A recent paper published by Hakan Duman (Recep Tayyip Erdoğan University, Rize, Turkey) and colleagues in the Journal of Vascular Surgery: Venous and Lymphatic Disorders has concluded that lower levels of bilirubin were significantly associated with venous thromboembolism (VTE),...
Michael D Dake (Tucson, USA) presented results from the VERNACULAR trial, which was designed to assess the performance of the Venovo venous stent (BD) for the treatment of iliac and femoral vein occlusions. At one year, the researchers found that the...
Mahmood Razavi (Orange, USA) reported new data from a subanalysis of the VIRTUS trial, which set out to investigate the safety and efficacy of the Vici stent (Boston Scientific). The subanalysis forms part of the first prospective multicentre corelab...
Kasuo Miyake is a leader in Cryo-Laser and Cryo-Sclerotherapy (CLaCS) treatments for varicose veins. Based in Sāo Paulo, Brazil, Miyake heads the vein clinic founded by his late father, Hiroshi Miyake, a pioneer in the field of CLaCS and...
Healogics, a provider of advanced chronic wound care services in the USA, has announced they have added vein ablation to their in-house service offerings through a new programme called Healogics VeinCare. This programme allows the company to provide patients...
BD has announced that the US Food and Drug Administration (FDA) has granted premarket approval for the Venovo venous stent, the first stent indicated to treat iliofemoral venous occlusive disease.
The Venovo venous stent is a flexible nitinol stent specifically...
The CASSINI trial found that treatment with rivaroxaban did not significantly reduce the incidence of thromboembolism or death caused by thromboembolism in high-risk ambulatory patients with cancer during the 180-day study period. However, during the study’s intervention period, there...
The commissioner of the US Food and Drug Administration (FDA), Scott Gottlieb, has unexpectedly resigned after serving just shy of two years in the post.
Gottlieb announced his intention to stand down in a letter to Alex Azar II, the...
At the 2018 Vascular Societies’ Annual Scientific Meeting (VSASM; 27–29 November, Glasgow, UK), Bruce Campbell from Exeter Medical in Exeter, UK, who previously chaired National Institute for Health and Care Excellence (NICE) advisory committees for interventional procedures and medical...
Johann Chris Ragg speaks to Venous News about the innovations he pioneered and the work that inspired his projects, as well as the challenges and benefits of running a private vascular clinic specialising in endovenous treatments. Ragg recounts his...
In this issue:
New ATTRACT data show greater benefits of catheter-based therapy for iliofemoral DVT patients
NICE aortic guidelines debate prompts scrutiny of venous guidelines and evidence gaps
Thom Rooke: Avoiding sclerotherapy complications
Profile: Johann Chris Ragg
In this issue:
New ATTRACT data show greater benefits of catheter-based therapy for iliofemoral DVT patients
NICE aortic guidelines debate prompts scrutiny of venous guidelines and evidence gaps
Thom Rooke: Avoiding sclerotherapy complications
Profile: Johann Chris Ragg
The UK's National Institute of Health and Care Excellence (NICE) has published new guidance recommending UrgoStart wound dressings (Urgo Medical) for treatment of diabetic foot ulcers and venous leg ulcers. In a press release, Urgo Medical states that use...
The editors-in-chief of major cardiovascular journals—of both US and European societies—have come together to “sound the alarm” about the dangers of medical misinformation that has been disseminated through the internet, social media, and other platforms. They claim that this...
Philips launches its Azurion with FlexArm to aid patient imaging and positioning during image-guided procedures.
During increasingly complex interventions, clinicians need to quickly and easily visualise critical anatomy and identify changes to the patient during the procedure. Azurion with FlexArm...
Medical device company Hancock Jaffe has announced the end-points for its upcoming VenoValve first-in-human study in Bogota, Colombia. Endpoints for the study will include improvements in reflux time, as well as three venous clinical measurement tools: the revised Venous...
Speaking at the Leipzig Interventional Course (LINC; 22–25 January, Leipzig, Germany), Katja Mühlberg of the University of Leipzig, Germany, called for an improvement in the evidence base around the role of compression in healing venous leg ulcers (VLU). Mühlberg...
New 12-month data from the VIRTUS trial demonstrates that patients who were treated with the Vici Venous Stent system (Boston Scientific) for iliac and femoral vein obstructions exhibited a high rate of patent, or open, target lesions. Primary safety and...
The US government shutdown means the country’s Food and Drug Administration (FDA) cannot accept new user fees, which means the agency cannot accept new medical product applications.
FDA commissioner Scott Gottlieb took to Twitter to highlight agency employees who are...
The first-in-human case using the new deep vein thrombosis (DVT) device Vetex thrombectomy catheter (Vetex Medical) has recently been completed in a multicentre study. The device has the potential to reduce hospital stays and costs associated with DVT treatment,...
A single-centre experience using large, braided self-expanding stents for iliofemoral venous obstruction found "excellent" outcomes. Several useful techniques and pearls for improved outcomes are reported in the January edition of the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
Placement...
Merit Medical Systems, Inc. has announced that it has acquired substantially all of the assets of Vascular Insights, LLC, based in Quincy, USA. Vascular Insights’ primary assets are the ClariVein IC and ClariVein OC specialty infusion and occlusion catheter...
The study data were delivered in two presentations at VEITHsymposium (13–17 November, New York, USA) by Mark Adelman (NYU Langone Hosptial, New York, USA) and Kathleen Gibson (Lake Washington Vascular Surgeons, Bellevue, USA).
They explained that incompetent perforator veins are...
An analysis of ATTRACT has recently been published in the journal Circulation, in which the authors examine the effect of pharmacomechanical catheter-directed therapy (PCDT) in ATTRACT patients with iliofemoral deep vein thrombosis (DVT). As a result of this, Venous News spoke to...
Venous News arranged a roundtable with Sophie Renton (London, UK), Bruce Campbell (Exeter, UK) and Manj Gohel (Cambridge, UK), who called for more high quality, medium-to-long-term evidence to support the growing use of minimally invasive, but expensive, interventions for deep...
The members of the American College of Phlebology (ACP) have voted to change their name to the American Vein and Lymphatic Society (AVLS). The name change is a part of the Society’s mission and vision to provide advocacy efforts,...
A new study, recently published online in the journal Circulation, reports on outcomes from a subgroup of 391 patients with acute iliofemoral deep venous thrombosis (DVT) in whom pharmacomechanical catheter-directed thrombolysis (PCDT) was evaluated within the ATTRACT trial.
The authors,...
Kathleen Gibson (Bellevue, USA), one of the principal investigators of the SeCure trial, presented its results at the ACP 2018 annual congress. “More than closure rates, it is the quality of life findings from this trial that are important...
Inari Medical has announced the enrolment of the first patient in the FlowTriever All-Comer Registry for Patient Safety and Hemodynamics (FLASH) using the company’s FlowTriever System for the treatment of pulmonary embolism (PE).
FLASH is a 500-patient prospective, multicentre registry...
Results from the Phase 3 CASSINI study contributes new data on the use of oral anticoagulant rivaroxaban (brand name Xarelto, Johnson & Johnson) in the management and prevention of venous thromboembolism (VTE) in high-risk patients with cancer.
The composite...
The world’s first bioconvertible IVC filter commercially offered in the USA (Sentry; from BTG) has been successfully implanted into the first patients outside of a clinical trial.
The Sentry filter is designed to provide protection from pulmonary embolism for the...
In this issue:
Strategies needed to address radiation exposures during venous procedures
Nationwide study finds no significant difference between rivaroxaban and apixaban for VTE patients
Profile: Ramesh Tripathi
Una adderley: Choosing the right compression
Alison Hopkins: Changing the narrative...
In this issue:
Strategies needed to address radiation exposures during venous procedures
Nationwide study finds no significant difference between rivaroxaban and apixaban for VTE patients
Profile: Ramesh Tripathi
Una adderley: Choosing the right compression
Alison Hopkins: Changing the narrative...
The US FDA has announced plans to modernise its 510(k) clearance programme for approving medical devices for the US market. Data show that about 20% of current 510(k) devices are approved on trials that compare novel devices to predicate...
Having worked as a vascular surgeon and venous specialist in several different countries and continents, Ramesh Tripathi has a wide-ranging experience and storied career. He tells Venous News about his current areas of research, his opinion of the most important...
Boston Scientific has announced it has reached an agreement on the terms of a recommended offer to acquire BTG, a company headquartered in the United Kingdom, which develops and commercialises products used in minimally-invasive procedures targeting cancer and vascular...
Two-year results for the SENTRY trial were presented at the Vascular Interventional Advances conference (VIVA; 5–8 November, Las Vegas, USA). The prospective, multicentre trial of the Sentry (BTG), a bioconvertible IVC filter, found that in addition to providing protection...
The 12-month results from the VERNACULAR trial have shown that the Venovo venous stent (BD Interventional) is successful when deployed in obstructive iliac and femoral lesions with a primary patency rate of 88.3%, a low reintervention rate of 7.4%,...
BTG has announced that the first EKOS Control Unit 4.0 have been shipped from BTG’s facility in Bothell (Washington, USA) to Europe, where full commercial launch will begin. New features of the EKOS CU 4.0 include an interactive colour...
There is a robust evidence base to support the use of compression therapy for healing venous leg ulceration and for preventing ulcer recurrence. The most recent version of the Cochrane Review1 on the effectiveness of compression and the relative effectiveness of different...
Theraclion has announced that Alfred Obermayer, principal investigator of the varicose vein treatment clinical trial in Austria, presented preliminary results at the Congress of the German Society of Phlebology in Bielefeld, attended by around 1,200 participants. The study aims...
At the Royal Society of Medicine (RSM) Venous Forum meeting (25–26 June, London, UK), Alison Hopkins was asked to speak about managing painful leg ulcers. She took the opportunity to focus on the need to improve the art of...
A recent Danish study, examining a nationwide cohort of 8,187 patients with venous thromboembolism (VTE) treated with one of two new oral anticoagulant therapies, reports no significant differences between the drugs in risk of all-cause mortality, recurrent VTE or...
Research presented at the European Society for Vascular Surgery’s annual meeting (ESVS; 25–28 September, Valencia, Spain) has shone a light on the potentially high cumulative radiation exposure associated with certain venous procedures. Addressing the issue, Stephen Black (Guy’s and...
In this issue:
"Crystal clear" evidence that higher compression reduces ulcer recurrence
New intravascular ultrasound-based scoring system may predict stent failure
Managing superficial vein thrombosis
Profile: Kathleen Gibson
This article is an advertorial sponsored by plus medica
The blueflow Venous Stent developed by plus medica GmbH & Co. KG received CE mark in early 2018, and has now been used in 60 patients at two centres in Europe....
A recent study which set out to investigate the usefulness of duplex ultrasound in monitoring stent changes over time, concludes that stent occlusion is related to the reduction of stent lumen over time—rather than the percent of the stenosis. Investigators...
Peripartum women with deep vein thrombosis (DVT) treated with catheter-directed thrombolysis and venous stenting had significantly increased rates of reintervention and loss of stent patency compared with non-pregnant women treated at the same centre.
Katalin Lestak (St Thomas’ Hospital, London, UK) presented...
A report of an eight-year experience using diode laser for the treatment of congenital diffuse venous malformations, has shown the modality to be effective and safe with a low number of complications.
The experience from the Vascular Anomalies Unit in...
A study presented at the European Society for Vascular Surgery’s annual meeting (ESVS; 25–28 September, Valencia, Spain) has concluded that mechanochemical ablation with concomitant ambulatory phlebectomy (MOCAP) results in greater early improvement in quality of life and significant reduction...
An accomplished vascular surgeon and prominent clinical researcher in the venous field, Kathleen Gibson (Lake Washington Vascular Surgeons, Bellevue, USA) plays an active role in a range of venous and vascular societies, and is currently involved in developing standardisation...
In this issue:
"Crystal clear" evidence that higher compression reduces ulcer recurrence
New intravascular ultrasound-based scoring system may predict stent failure
Managing superficial vein thrombosis
Profile: Kathleen Gibson
Anna Louise Pouncey revealed at the European Venous Forum annual meeting (EVF; 28–30 June, Athens, Greece) the findings of the GSTT venous team (Guy’s and St Thomas’ NHS Foundation Trust & Kings College London, London, UK) that early use...
Superficial vein thrombosis is not a rare disease, with an incidence of 0.64 in 1,000 annually.1 The POST, OPTIMEV and STEPH studies have shown that superficial vein thrombosis may be associated with a considerable risk of concomitant deep vein...
The utility of combining patient- and physician-reported scores to stratify disease severity and treatment rationale in patients with varicose veins has been highlighted in a recent study by Lowell Kabnick, Thomas Wakefield, Mikel Sadek, Jose Almeida and Glenn Jacobowitz,...
BTG has announced it has acquired Novate Medical, a medical device company focused on the prevention of pulmonary embolism in patients at high risk of venous thromboembolic events.
Novate has developed Sentry, the first bioconvertible inferior vena cava filter, which...
Mermaid Medical Group, a privately-owned international provider of minimally invasive medical devices, acknowledged a product line addition to its vascular business. As part of its strategic focus for expanding their vascular product portfolio, Mermaid Medical Group announced the acquisition...
Alex C Spyropoulos, from Feinstein Institute for Medical Research (New York, USA), has presented an anticoagulant treatment strategy to reduce non-fatal blood clots and pulmonary embolism in acutely-ill hospitalised patients, according to clinical findings published in The New England...
One of the first randomised controlled trials comparing two therapeutic regimes—surgery and foam sclerotherapy—in patients with anterior accessory saphenous vein (AASV) varicosis, has found that surgery can outperform foam sclerotherapy in terms of duplex sonographic recurrence after one year....
To accompany the growing importance of intravascular ultrasound (IVUS) as an adjuvant diagnostic tool when treating deep venous disease, a study sought to create an IVUS-based scoring system able to predict venous stent failure in the treatment of May-Thurner...
Hancock Jaffe Laboratories, a company specialising in bioprosthetic medical devices for treating cardiac and vascular diseases, has received approval for the first-in-man testing of its VenoValve bioprosthetic venous valve device from the Medical Research Committee at Fundación Santa Fe...
According to Fedor Lurie (Jobst Vascular Institute, Toledo, USA), who presented at the European Venous Forum Annual Meeting (EVF; 28–30 June, Athens, Greece), patients with a high probability of iliofemoral deep vein thrombosis (DVT) are not diagnosed early enough...
A first prototype of a novel endovenous therapy technology (SnakeBack, LSO Medical) integrated with a laser ablation platform was revealed earlier this year at the Charing Cross Symposium (CX; 24-27 April, London, UK) by Philippe Rochon, president of LSO...
Boston Scientific has signed an agreement to acquire Veniti, a privately-held company in Fremont, USA which developed and commercialised the Vici venous stent system for treating venous obstructive disease. Boston Scientific has been an investor in Veniti since 2016 and...
NexGen Medical Systems, Inc., a US medical device company, has announced the successful completion of the first human use of their XCOIL large vessel (18mm) thrombectomy system for the treatment of deep vein thrombosis (DVT). The new device, an expansion...
A new analysis has identified patient populations with cancer-associated venous thromboembolism (VTE) who could benefit from treatment with oral anticoagulant edoxaban, taken once daily.
Daiichi Sankyo has announced the publication of a new analysis, focusing on the clinical presentation, course...
A Cambridge study has found that most inpatients with leg ulcers were not referred to a vascular surgery team, indicating a need to improve pathways of care for inpatients with leg ulcerations.
The results of the study conducted at Addenbrooke’s...
Venclose has announced the closing of an oversubscribed Series B round of financing from new and existing investors. Proceeds from the financing will be used to support new product development, expansion of manufacturing capabilities and general corporate purposes.
The company...
EchoNous Vein (EchoNous), an ultrasound-based tool designed specifically for nurses to improve peripheral IV catheter placements, has received US Food and Drug Administration (FDA) approval.
A company release explains that EchoNous Vein provides immediate, clear images at depths from 1–5cm...
Alun Davies is a Welsh-born leading expert in the venous field, one of the Editors-in-Chief of Venous News, and currently in the spotlight as chief investigator of the EVRA ulcer trial. In this profile, Davies talks about the implications...
Highlights:
Landmark EVRA trial provides first Level 1 evidence for early venous ablation
Ultrasound-guided foam sclerotherapy of small saphenous vein as effective as endovenous laser ablation for venous clinical severity score and quality of life at one year
Rick...
Highlights:
Landmark EVRA trial provides first Level 1 evidence for early venous ablation
Ultrasound-guided foam sclerotherapy of small saphenous vein as effective as endovenous laser ablation for venous clinical severity score and quality of life at one year
Rick...
An analysis of post-thrombotic disease patients who received a nitinol stent has found that ultrasound surveillance should occur at frequent intervals up to two weeks post-procedure to predict risk of re-intervention.
The results of the study were presented at the...
A new randomised controlled trial evaluated the preventative effect of compression stockings during pregnancy on the incidence of varicose veins as well as venous thromboembolism, with results suggesting that women with no venous disease using compression stocking during pregnancy...
BTG has announced a strategic partnership with the PERT Consortium to advance the science of pulmonary embolism treatment and promote the implementation of PERT programmes across the USA. The partnership was announced at the annual PERT Consortium Meeting (22–23...
The annual census of UK consultants and higher speciality trainees—Focus on Physicians 2017–18—indicates that more than half of all consultants and two thirds of trainees reported frequent gaps in trainees’ rotas, with one in five respondents saying these are...
The one-year results of the ACCESS PTS trial have shown that EKOS therapy (BTG) statistically improves post-thrombotic syndrome scores and sequelae as well as quality of life outcomes. Presenting the data at the Charing Cross Symposium (24–27 April, London,...
Cees Wittens writes for Venous News about the current state of deep venous obstruction interventions, what we might expect in the near future, and what is needed in terms of new technology, strategy and policy in 2018.
In general, everybody...
Treatment of patients with pelvic venous disorders is a “controversial topic,” Kathleen Gibson (Lake Washington Vascular Surgeons, Bellevue, USA) said at the Charing Cross Symposium (CX; 24–27 April, London, UK), as there is “no consensus on the best mode...
New data from the MERCURY PE study have shown that patients with low-risk pulmonary embolism who were treated with the direct oral anticoagulant rivaroxaban, trade name Xarelto (Janssen Pharmaceutical), and discharged from the emergency department had significantly reduced time...
St Paul's Hospital in Vancouver, Canada has successfully completed two implantations of the VenusP-Valve (Venous Medtech), a self-expanding pulmonary valve. This is the first appearance of Venus Medtech's transcatheter valve products in North America.
Although surgical treatment of congenital heart disease has evolved...
Major global wound care company Acelity is acquiring Crawford Healthcare, a growing UK-based advanced wound care and dermatology company, along with all of its assets. The companies announced the acquisition today, but did not disclose the terms of the...
Countries in the European Union have long been the first to receive new innovations in medical technology, as the EU’s Medical Device Directive (MDD) provided quicker routes to implementation of new devices than its equivalent in the USA, the...
Data presented at the Charing Cross Symposium (24–27 April, London, UK) indicate that a rounder post-stent lumen shape has a positive correlation to 12-month patient improvement, placing emphasis on the change of lumen shape from pre- to post-stent and...
Vesper Medical, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, has announced that it completed its Series A financing, totaling US$10.5 million. Major participants in the Series A financing included New Enterprise Associates and Quaker...
Inari Medical have announced the presentation of results from its FlowTriever Pulmonary Embolectomy (FLARE) clinical study that evaluated the safety and effectiveness of the FlowTriever Retrieval/Aspiration System for the treatment of pulmonary embolism. The results were presented by Thomas Tu, an...
According to a debate at the Charing Cross Symposium (CX; 24–27 April, London, UK), the vast majority of CX delegates disagree with the view that the ATTRACT trial has provided the definitive answer on the use of pharmacomechanical catheter-directed...
For many venous specialists, dedicated venous stents represent the future—and the promise of an ideal venous stent on the horizon is a central topic of discussion. In this article, Rick de Graaf reviews the recent history of venous stenting...
Preliminary data from the Fovelass trial, presented at the Charing Cross Symposium (24–27 April, London, UK) by Claudine Hamel-Desnos (Caen, France) show that at one-year follow-up, the rate of occlusion of the small saphenous vein is lower after ultrasound...
The first full results of the Early Venous Reflux Ablation (EVRA) ulcer study were presented at the Charing Cross Symposium (CX; 24-27 April, London, UK). The presentation was accompanied by simultaneous publication in the New England Journal of Medicine...
Cardiva Medical, a vascular closure device company, today announced it has received approval from the US Food and Drug Administration (FDA) for an expanded indication of the VASCADE Vascular Closure System. Already approved for use in arterial closure, the...
The Royal Society of Medicine Venous Forum council has created a document that is intended to aid physicians when treating patients with leg ulcers.
The document, “Management of Patients with Leg Ulcers”, indicates that urgent action is needed to ensure that...
Intravascular ultrasound (IVUS) imaging technology provides the current gold standard technique for detection and characterisation of venous disease, Erin Murphy writes. In this article, Murphy discusses the limitations of venography and outlines the use of IVUS for optimised outcomes...
Venture-backed medical device company Inari Medical has announced the close of a Series C financing totaling US$27 million. The company plans to use the capital to commercialise its catheter devices for the treatment of venous thromboembolism.
The financing was led by new...
Medical device company Plus medica has announced that 10 patients have been treated with the company’s blueflow Venous Stent. The first cases were performed by Michael Lichtenberg, Klinikum Arnsberg, Arnsberg, Germany, and Nils Kucher, University Hospital Zurich, Zurich, Switzerland.
The...
The handheld medical imaging system HyperView (HyperMed Imaging) has received CE mark, allowing for distribution in Europe. The system is designed to detect perfusion of superficial tissue in support of wound care.
The HyperView System is a fast, handheld, battery...
Representation is essential in the constantly changing medical landscape that healthcare professionals are facing. The American College of Phlebology (ACP) has begun several new initiatives recently to improve the representation of venous and lymphatic health care providers within the...
The deep venous world is wrestling with the question: To stent or not to stent. While the availability of new specialised venous stents is boosting popularity for the technique, there are some reservations about the appropriateness and risks of...
Following the announcement of receiving the CE mark in early 2018, ab medica has now reported the first-in man usage of its blueflow Venous Stent. The company signed an exclusive distribution agreement for the blueflow Venous Stent with plus medica...
The common diagnosis of post-thrombotic syndrome (PTS) includes a history of deep venous thrombosis and a Villalta Scale score of four or above. However, a recent study conducted by JOBST Vascular Institute of ProMedica, examining whether misclassification bias might occur...
The International Union of Phlebology World Congress (UIP) will head to Turkey in 2021 after Istanbul successfully won the bid to be the host city of the next meeting.
The battle for the host city of UIP 2021 was hotly...
Highlights:
CX presents comprehensive Venous Controversies programme
UIP audience votes against stenting as standard for deep vein obstruction
Setting the record straight: Clarifying the misconceptions of the ATTRACT trial
Erin Murphy: Use of IVUS to optimise outcomes in venous...
Highlights:
CX presents comprehensive Venous Controversies programme
UIP audience votes against stenting as standard for deep vein obstruction
Setting the record straight: Clarifying the misconceptions of the ATTRACT trial
Erin Murphy: Use of IVUS to optimise outcomes in venous...
Rick de Graaf speaks to Venous News about complications from venous stenting, including causes and best treatment approaches for reinterventions after stenting.
A special session during the pre-conference Day of Innovation and Science at the American Venous Forum annual meeting (AVF; 20–23 February, Tucson, USA) presented the rare occasion for attendees to listen to and interact directly with the principal investigator...
A recent study on iliac vein stenting following catheter-directed thrombolysis for deep vein thrombosis (DVT) has shown that technical and clinical success require high thrombus clearance rates, and stenting below the inguinal ligament does not have an adverse outcome...
After five years, the board of the European College of Phlebology is changing, announced Eberhard Rabe (University of Bonn, Bonn, Germany) at the European Vascular Course (EVC; 4–6 March, Maastricht, The Netherlands). The election is to be held among...
The blueflow Venous Stent (plus medica) received the CE mark in January 2018.
The news was announced via a company release in March.
The blueflow Venous Stent, developed and manufactured in Germany is a two nitinol wire braided stent used to...
UK-based Inotec AMD Limited has announced the appointment of Craig Kennedy as its new chief executive officer on 3 March 2018.
Inotec AMD is a maker of mobile medical devices designed to heal chronic, hypoxic wounds that afflict millions of...
At the American Venous Forum (AVF; 20–23 February, Tucson, USA), a session on venous leg ulcers turned to possible future treatment strategies as Kathleen Gibson (Lake Washington Vascular Surgeons, Bellevue, USA) spoke about off-label uses of cyanoacrylate and polidocanol...
Efficacy data from the Arnsberg Registry at 12 months are promising, with high primary patency rates for a dedicated venous stent.
Presenting the data at the Leipzig Interventional Course (LINC; 30 January–2 February, Leipzig, Germany), the registry’s principal investigator Michael...
Armando Mansilha is professor of Angiology and Vascular Surgery, Faculty of Medicine at the University of Porto, Porto, Portugal. In this interview with Venous News, he speaks about his early work and mentors, the biggest developments in venous medicine...
The Food and Drug Administration (FDA) has today issued the final rule on “human subject protection; acceptance of data from clinical investigations for medical devices”.
The rule updates the FDA’s standards for accepting clinical data from clinical investigations conducted...
“Endovascular reconstruction of the chronically obstructed iliocaval venous tract with stents is technically feasible, minimally invasive and has an acceptable safety rate, said James Budge, Department of Vascular Surgery, Guy’s and St Thomas’, London, UK.
“Patients who underwent this procedure...
Following the publication of the ATTRACT trial in the New England Journal of Medicine (NEJM) in December 2017, Venous News spoke to experts in the field to analyse the data and formulate future research questions. One of the interesting...
Highlights:
Published ATTRACT data call into question validity of the Villalta scale
Brave Dreams trial finds venoplasty “safe, but largely ineffective” for MS patients
Johann C Ragg: Percutaneous valvuloplasty
Nicholas Osborne: VQI Varicose Vein Registry
Profile: Armando Mansilha
https://venousnews.com/wp-content/uploads/sites/19/2018/02/Venous-News-4-low-res-US.pdf
Highlights:
Published ATTRACT data call into question validity of the Villalta scale
Brave Dreams trial finds venoplasty “safe, but largely ineffective” for MS patients
Johann C Ragg: Percutaneous valvuloplasty
Nicholas Osborne: VQI Varicose Vein Registry
Profile: Armando Mansilha
https://venousnews.com/wp-content/uploads/sites/19/2018/02/Venous-News-4-low-res-EU.pdf
LimFlow has announced completion of enrolment of the original 10-patient cohort in the US feasibility study of the LimFlow percutaneous deep vein arterialisation system.
The company also announced that the US FDA has accepted the company’s proposal to expand...
Medtronic has announced the initiation of its investigational device exemption (IDE) study for the Abre venous self-expanding stent system. The ABRE IDE Study will evaluate the safety and effectiveness of the Abre stent in patients with iliofemoral venous outflow...
Members of the venous community have had a longstanding commitment to collecting and analysing the outcomes of venous procedures. The Vascular Quality Initiative (VQI) Varicose Vein Registry (VVR) grew out of a previous registry established by the American Venous...
Venous specialists from around the globe will meet in Melbourne for the much anticipated 2018 World Congress of Phlebology (UIP; 4–8 February, Melbourne, Australia) hosted by the Australasian College of Phlebology.
The president of UIP (International Union of Phlebology), Nick...
Compression therapy for venous ulcers requires accurate monitoring to ensure successful delivery and the desired compression dose. However, according to a study presented at the American College of Phlebology annual meeting (ACP; 2–5 November, Austin, USA), the level of...
The Janssen Pharmaceutical Companies of Johnson & Johnson has announced findings from the US Food and Drug Administration's (FDA) mini-sentinel assessment, confirming the positive safety and efficacy profile of Xarelto (rivaroxaban) established in the phase III ROCKET AF clinical trials,...
Daiichi Sanyoko has announced the results from their Hokusai-VTE CANCER trial evaluating the direct oral anticoagulant edoxaban for treatment of venous thromboembolism (VTE) in cancer patients.
The Hokusai-VTE CANCER study is a phase 3b, prospective, randomised, open-label, blind end-point study...
The European Board of Phlebology has announced that the European Union of Medical Specialists (UEMS) have adopted the European training requirements presented by Jean-Jérôme Guex, president of the newly created Board. The development is the result of a European...
Global specialist healthcare company BTG has highlighted the commencement of the KNOCOUT PE study. The KNOCOUT PE study will measure how hospitals and patients are benefitting from a new standard of care in the treatment of pulmonary embolism utilising...
Venous stenting has evolved over the last 15 years, quickly becoming an established procedure, with Class II recommendations from the American Heart Association and American College of Phlebology. Speaking at the Vascular Society of Great Britain and Ireland’s Annual...
Leg ulceration is the poor relation of vascular disease, yet, as Sophie Renton writes, it is a very common problem. It is estimated that 1/1,000 people in the UK will have a leg ulcer at some stage in their lives. Leg ulcers become...
More than 25% of the adult population is affected by varicose veins, with more than a 15% prevalence of chronic venous insufficiency (C3–C6), which induces a considerable burden on the health of these patients. The Bonn Vein Study found that 21% of the...
At the THE VEINS at VIVA meeting (10–11 September, Las Vegas, USA), Esther Kim (Vanderbilt University Medical Center, Nashville, USA) gave a presentation on venous thrombosis in unusual locations, noting that such thromboses tend to occur in younger patients and those with malignancy,...
At THE VEINS at VIVA meeting (10–11 September 2017, Las Vegas, USA), James Froehlich, University of Michigan, Ann Arbor, USA, reviewed guidelines on the management of superficial venous thrombosis (SVT) and emphasised the importance of performing duplex ultrasound in the examination, assessing clot location...
Cardiff Metropolitan University has announced it has secured a grant to “support an interdisciplinary collaboration of an innovative sterilising device for venous access ports (VAPs).”
Existing VAPs tend to encourage the build-up of bacteria and fungus in the port, leading...
BTG has announced its finalised category I CPT codes, received from the US Centers for Medicare and Medicaid Services. The codes will be be effective from 1 January, 2018.
The fee schedule details payments for conducting Varithena treatments in the...
Cardiff Metropolitan University has successfully secured funding to support an interdisciplinary collaboration arising from the Welsh Crucible programme that looks at the design and development of an innovative sterilising device for venous access ports (VAPs).
The interdisciplinary research is a...
The US Centers for Medicare and Medicaid Services (CMS) has posted the 2018 Medicare Physician Fee Schedule (CMS-1678-F) and Hospital Outpatient Prospective Payment and Ambulatory Surgical Center Payment Systems (CMS-1678-FC). As part of the final rulings, healthcare providers will...
Nick Morrison chose venous medicine as he wanted a challenging career. Although he initially worried that he would become bored working in the venous field, his long and distinguished career since then is proof of the complexity and depth of...
Continuing work to develop an improved percutaneous valvuloplasty procedure “is promising”, said Johann Chris Ragg (Angioclinic Vein Centers, Berlin, Munich, Germany, and Zurich, Switzerland), as he updated delegates on the technique at the annual American College of Phlebology meeting...
Inferior vena cava (IVC) filters have been a highly controversial topic in the field of venous medicine due to the high rates of complications associated with their use, which eventually prompted a 2010 US Food and Drug Administration (FDA) advisory and declining...
Highlights: -MOCA and EVLA show similar overall pain scores and improvements in quality of life and clinical outcomes -First European advice on deep vein thrombosis published in European Heart Journal -Armando Mansilha: Chronic venous disease -Sophie Renton: Venous ulceration guidelines -Profile:...
Highlights: -MOCA and EVLA show similar overall pain scores and improvements in quality of life and clinical outcomes -First European advice on deep vein thrombosis published in European Heart Journal -Armando Mansilha: Chronic venous disease -Sophie Renton: Venous ulceration guidelines -Profile:...
While comparative study data for venous stents are still lacking, results of a number of ongoing and planned venous stent trials will increase the options currently available to physicians and provide useful comparative data, explained Stephen Black (London, UK),...
Endovascular treatment of iliocaval and infrainguinal post-thrombotic venous obstruction results do not appear to be adversely affected by extension of the iliac vein stents into the femoral venous system, according to a US report in the Journal of Vascular...
Janssen Pharmaceuticals has announced that the FDA has approved the 10mg once-daily dose of rivaroxaban (Xarelto) for reducing the continued risk for recurrent venous thromboembolism (VTE) after completing at least six months of initial anticoagulation therapy.
The approval follows a...
Inari Medical has announced that it has completed enrolment of its investigational device exemption (IDE) study. The FlowTriever Pulmonary Embolectomy Clinical Study (FLARE) study is designed to evaluate the safety and effectiveness of the FlowTriever Retrieval/Aspiration System for the...
Deep venous stenting has become increasingly used to treat patients with pathological obstruction of venous return in the femoro-iliocaval venous segment following the development of new dedicated venous stents. Patients treated range from those with an isolated non-thrombotic iliac vein lesion, to those...
Marianne De Maeseneer already wanted to become a doctor by the age of 12, with her imagination captured by the idea of working in developing countries. A “series of coincidences” led her towards vascular and venous medicine, in which...
The first comprehensive European advice on deep vein thrombosis was recently published in the European Heart Journal. The recommendations were produced by the European Society of Cardiology (ESC) Working Group on Aorta and Peripheral Vascular Diseases and Working Group...
The world’s largest randomised trial comparing three central venous access devices—peripherally inserted central catheters (PICCs), Hickman-type devices and chest wall ports—should provide definitive results in terms of their relative efficacy and cost-effectiveness, Jon Moss, Glasgow, UK, told CIRSE delegates.
The...
Gerard Goh, Radiology, The Alfred Hospital, Melbourne, Australia, reported results from a comparative study of inferior vena cava (IVC) filters at CIRSE 2017 that examined the effectiveness, safety and complication rates of the Celect, Celect Platinum and ALN vena...
Data from the LAMA (Laser ablation versus mechanochemical ablation) trial indicate that mechanochemical ablation (MOCA) is significantly less painful than endovenous laser ablation (EVLA) during treatment of superficial venous incompetence, though there is no difference in pain between the...
There are two things you need to know about me. Firstly, I am a health psychologist, with a specialist interest in behaviour change, implementation, and impact. Secondly, through a series of deep vein thromboses (DVTs) beginning with a post-partum iliofemoral clot in 2008...
Emerging evidence suggests that the lymphatics play an important role in the early pathogenesis and progression of peripheral arterial and venous diseases, suggest John Rasmussen and Eva Sevick-Muraca, writing for Venous News.
Functioning peri-adventitial lymphatics are essential for reverse cholesterol transport by macrophages to...
Although the venous stent is still a young technology, it is already offering new and exciting opportunities to patients with deep venous occlusive disease. Venous News spoke to Manjit Gohel (Cambridge, UK) about how his experience in with deep venous stenting,...
ElastiMed has raised US$1 million from a strategic investor, existing investors—The Trendlines Group and Pix Vine Capital—new private investors, and the Israeli Innovation Authority. This represents the company's second round of funding, bringing total raises to US$2 million to...
Inferior vena cava (IVC) filters have long been a controversial topic, and some physicians have even called for a total moratorium on their implantation. Venous News spoke to John Kaufman (Portland, USA) at the 2017 Vascular Interventional Advances 2017...
A study has shown modified human factor X to be a safe and effective reversal agent for prevention and treatment of bleeding in patients taking factor Xa oral anticoagulants. This new therapeutic factor X was inspired by a snake...
Twelve-month results from the SENTRY trial evaluating the Sentry bioconvertible inferior vena cava (IVC) filter (Novate) have shown a 0% rate of symptomatic pulmonary embolism, no instances of device failure and a 96% rate of bioconversion. Presenting that data...
Spectranetics has recalled the Bridge occlusion catheter “due to the possibility of a blocked guidewire lumen in some device units”. Using affected catheters could lead to the incorrect positioning of the device and subsequent uncontrolled haemorrhage.
Spectranetics’ Bridge occlusion balloon...
Highlights: -Five-year data find foam sclerotherapy to be less effective than thermoablation or stripping for varicose veins -Questions remain over venous leg symptoms despite SYM vein consensus -Manjit Gohel: Stent flexibility -Eva Sevick-Muraca: Lymphoedema -Profile: Marianne De Maeseneer
https://venousnews.com/wp-content/uploads/sites/19/2017/09/Venous-News-2-low-res_USA.pdf
Highlights: -Five-year data find foam sclerotherapy to be less effective than thermoablation or stripping for varicose veins -Questions remain over venous leg symptoms despite SYM vein consensus -Manjit Gohel: Stent flexibility -Eva Sevick-Muraca: Lymphoedema -Profile: Marianne De Maeseneer
https://venousnews.com/wp-content/uploads/sites/19/2017/09/Venous-News-2-low-res.pdf
ManaMed has introduced PlasmaFlow, the first US Food and Drug Administrator-approved portable and tubeless deep vein thrombosis (DVT) prevention device throughout the USA.
"We at ManaMed are thrilled that our innovative PlasmaFlow DVT prevention device is available to help mitigate...
Medtronic is expanding its embolisation product portfolio with the launch of the Concerto 3D detachable coil system. The system was launched at the Cardiovascular and Interventional Radiological Society of Europe annual meeting (CIRSE; 16–20 September, Copenhagen, Denmark).
The Concerto 3D...
InGeneron has announced the publication of results from an investigator initiated case series in chronic leg wounds in the Journal of the European Academy of Dermatology and Venereology.
In the case series, InGeneron’s proprietary adipose-derived regenerative cell (ADRC) technology was...
Boston Scientific will distribute Veniti’s Vici venous stent under a limited global distribution agreement. The terms of the agreement and specific regions and countries involved have not been disclosed.
Launched in 2014, the Vici venous stent has gained wide market...
Straub Medical has announced that the first patient has been enrolled in the P-MAX study at the Karolinen-Hospital Arnsberg, Germany.
The post-market clinical follow-up study to assess the safety and effectiveness of the AspirexS venous catheter in the treatment of deep vein thrombosis will enrol over 80 patients by...
Results from a Czech study indicate that 1470nm laser treatment of saphenous vein reflux with both radial single-ring and 2ring fibres results in clinical improvement of symptoms and comparable occlusion rates. That said, in the early postoperative period, 2ring laser...
At the 2017 European Venous Forum (29 June–1 July, Porto, Portugal), a presentation given by Eberhard Rabe (Bonn, Germany) questioned whether the SYM Vein Consensus clarified all aspects of venous symptoms. Speaking with Venous News, Rabe explains which questions still remain regarding venous leg symptoms...
The VIDIO study, led by Paul Gagne, Southern CT Vascular Center, Darien, USA, showed that intravenous ultrasound (IVUS) is more sensitive for assessing treatable iliofemoral vein stenosis compared with multiplanar venography and frequently leads to revised treatment plans and the potential for improved clinical outcome.
The...
The American College of Phlebology (ACP) board of directors has selected Dean Bender as the new executive director for the ACP and ACP Foundation.
Bender has more than 30 years in global business management and considerable experience in venous and lymphatic medicine. He has served as vice president of business...
Teleflex has announced a new three-year product category contract with Vizient for non-tunnelled central venous catheters. Vizient is the largest member-driven health care performance improvement company in the USA.
The new agreement for non-tunnelled central venous catheters, which became effective...
Medtronic has completed the previously announced sale of a number of its businesses to Cardinal Health, including its Deep Vein Thrombosis (compression) division. The transaction was worth US$6.1 billion.
The company has also sold its Patient Care and Nutritional Insufficiency...
Venous News caught up with Lars Rasmussen (Danish Vein Centres, Denmark) at the European Venous Forum (EVF; 29 June–1 July, Porto, Portugal) to hear about his five-year follow-up comparing endovenous radiofrequency ablation, laser ablation, foam sclerotherapy and surgical stripping...
Writing for Venous News, Paul Gagne (Darien, USA) notes that during the past decade, evaluation and treatment of iliac or common femoral vein obstructive disease have become more familiar and common. Based on the landmark work pioneered by Peter...
The first results from the GARFIELD-VTE (Global anticoagulant registry in the field—venous thromboembolism) were presented at the International Society on Thrombosis and Haemostasis Congress 2017 (8–13 July, Berlin, Germany) providing a contemporary picture of VTE management worldwide.
GARFIELD-VTE is a...
At the European Venous Forum (EVF; 29 June–1 July, Porto, Portugal), Mattia Mirandola (Peschiera del Garda, Italy) presented his four-year experience of using ClariVein (Vascular Insights) for mechanochemical ablation of the saphenous vein. He shared his results with Venous...
Endovenous adhesive closure systems are a useful adjunct to thermal ablation treatment for varicose veins, but it is vital that the possible side-effects of such treatments are discussed openly with patients pre-procedurally, according to a presentation at the European...
The UK’s Information Commissioner’s Office (ICO) has found that the Royal Free National Health Service (NHS) Foundation Trust (London, UK) did not adhere to the UK’s Data Protection Act when it provided patient details to Google DeepMind.
The Trust, a...
A presentation at the American Venous Forum (AVF; 14–17 February, New Orleans, USA) has attempted to shed light on what could be an underdiagnosed cause of pelvic pain caused by pelvic venous insufficiency—iliac vein compression.
Ratnam K N Santoshi (Center...
A study presented at the American Venous Forum (AVF; 14–17 February, New Orleans, USA) suggests that secondary interventions for primary iliac vein stenting are “associated with good outcomes,” with a rate of 10.2% reported.
Aiya Aboubakr (Mount Sinai Hospital, New...
Bio2 Medical has announced the results of the Angel Catheter pivotal study published in the Journal of Vascular and Interventional Radiology. The Angel Catheter met all safety endpoints and demonstrated a significant reduction in clinically significant and fatal pulmonary...
The US Food and Drug Administration (FDA) has accepted, for priority review, a supplemental new drug application (sNDA) for Janssen’s Xarelto (rivaroxaban), to include a 10mg once-daily dose for reducing the risk of venous thromboembolism (VTE) after at least...
Coming from a non-medical background, Keith Poskitt is unable to pinpoint exactly how his interest in the field developed. At school, science, and sports—rugby and cricket in particular—vied for his attention. Following a serious ankle injury at the age...
The US Food and Drug Administration (FDA) has approved betrixaban (Bevyxxa, Portola), the first and only anticoagulant for hospital and extended duration prophylaxis (35 to 42 days) of venous thromboembolism (VTE) in adult patients hospitalised for an acute medical...
Researchers at North Carolina State University (Raleigh, USA) and the University of North Carolina at Chapel Hill (Chapel Hill, USA) have developed a new surgical tool that uses low-frequency intravascular ultrasound to break down blood clots that cause deep...
Public Health England (PHE) and the Royal Society for Public Health (RSPH) have published “Everyday Interactions”, a report which aims to support healthcare professionals to record and measure their public health impact.
The report and toolkit were developed in close...
The results of the ACCESS PTS trial have been presented at the Society for Vascular Medicine 28th Annual Scientific Sessions (14–17 June, New Orleans, USA). The study found chronic deep vein thrombosis (DVT) patients with post-thrombotic syndrome (PTS) can...
New evidence suggests that minimally invasive methods to ablate superficial venous reflux in patients with end-stage venous insufficiency are as effective as traditional open venous stripping.
Chronic venous insufficiency affects approximately 2.5 million Americans, with up to 20% developing venous...
In this supplement: Life post-ATTRACT: The new challenge of DVT treatment --The future of DVT intervention in Europe: what needs to happen next --"With careful patient selection and a patent popliteal vein, DVT treatment can be completed in less...
Kathleen Gibson, Bellevue, USA, presented the three-year results of the VeClose clinical trial at the recent Charing Cross Symposium. Gibson spoke to Venous News about the study and about how she interprets the three-year data.
Results from the OPTALYSE PE trial have been presented at the American Thoracic Society International Conference in Washington, DC, USA. The results show that pulmonary embolism (PE) can be treated effectively with EKOS (BTG) over a shorter period and...
Highlights: -ATTRACT results are “a springboard” for future pharmacomechanical thrombolysis research -Paul Gagne: Stenosis threshold -Peter Gloviczki: A look into the future -Profile: Keith Poskitt
https://venousnews.com/wp-content/uploads/sites/19/2017/05/Venous-News-1-US_low-res.pdf
Highlights: -ATTRACT results are “a springboard” for future pharmacomechanical thrombolysis research -Paul Gagne: Stenosis threshold -Peter Gloviczki: A look into the future -Profile: Keith Poskitt
https://venousnews.com/wp-content/uploads/sites/19/2017/05/Venous-News-1-EU_low-res-1.pdf
At the American Venous Forum meeting (14–17 February, New Orleans, USA), Johann Christof Ragg (Berlin/Zurich, Germany/Switzerland), presented on the concept of obtaining ultrasound proof of pre-reflux stages of venous insufficiency. Ragg spoke to Venous News about what light his...
Data from the randomised controlled ATTRACT trial revealed that the addition of catheter-based intervention to standard-of-care anticoagulation failed to significantly decrease the occurrence of post-thrombotic syndrome in patients who received this treatment strategy when compared with its occurrence in...
Three-year outcomes from the VeClose US pivotal clinical trial and one-year data from the WAVES study have been presented at the 2017 CX Symposium in London, UK. Both results were presented by Kathleen Gibson (Bellevue, USA). The new data...
Isobar Compression has been awarded a research contract from the Small Business Research Initiative (SBRI) for Healthcare, to assist in a feasibly study in primary care for the treatment of venous leg ulcers.
The research contract was awarded by SBRI...
Bluegrass Vascular Technologies has enrolled over one third of its patients in the company's post-market SAVE (Surfacer System to Facilitate Access in Venous Occlusions) clinical study. The SAVE study is an international, prospective, multicentre clinical follow-up study designed to...
Research published in Phlebology has indicated that the 2013 UK varicose veins clinical guidelines have led to “a considerable increase” in leg ulcer patient referrals, although many patients are still not being referred early enough, the authors of the paper...
Inari Medical, a venture capital backed medical device company focused on the interventional treatment of venous thrombus, has announced the treatment of the first patient with its ClotTriever thrombectomy system.
The system received 510(k) marketing clearance from the US Food and...
Further evidence has been found by researchers at the UK’s University of Leicester and University of Bristol to suggest statins could "significantly reduce" the occurrence of blood clotting in certain parts of the body.
The research team analysed several studies...
Compression therapy is the mainstay of treatment for the symptoms of chronic venous insufficiency (CVI). CVI is treated with compression stockings or multilayer bandaging. However, as most compression stockings are supplied in standard sizes, they frequently failed to deliver...
The use of dedicated nitinol venous stents across the inguinal ligament shows “good outcomes” in terms of patency but reinterventions may be required, according to Prakash Saha, Guy’s and St Thomas’ NHS Foundation Trust and King’s College London, London,...
Most existing surgical options for symptomatic deep venous insufficiency (DVI) with venous stasis ulcers either fail or provide limited success. Many surgeons consider operating on large veins such as the common femoral vein to be foolhardy—something new is required,...
A Canadian-led international research team has found that the blood thinner rivaroxaban is as safe as aspirin, and more effective at preventing recurrence of life-threatening blood clots in the legs and lungs, according to a study published in the...
In a new study presented at the American College of Cardiology 66th Annual Scientific Session, researchers from the Perelman School of Medicine at the University of Pennsylvania have found that the utilisation rates of these potentially life-saving medications are low, particularly in...
Unfors RaySafe has introduced the RaySafe i3, to its suite of real-time dosimetry products, at the European Society of Radiology in Vienna.
The RaySafe Real-Time Dosimetry solution, introduced in 2012, helps physicians and clinical staff visualise X-ray exposure in real...
The use of computerised clinical decision support systems among surgical patients are associated with a significant increase in the proportion of patients with adequately ordered treatment to prevent blood clots, and a significant decrease in the risk of developing...
CorVascular has entered into a distribution agreement with Novarix. The IV-eye, developed and manufactured in the UK by Novarix, is a near infrared (NIR) vein imaging device designed to aid healthcare professionals in finding suitable peripheral veins for both cannulation...
Nobilis Health has closed its previously announced acquisition of Hamilton Vein Center.
“Hamilton Vein Center not only increases our in-network patient volume mix to over 60%, but its six facilities enhance our network of physicians, allowing Nobilis to generate additional...
Data from the randomised controlled ATTRACT trial revealed that the addition of catheter-based intervention to standard-of-care anticoagulation failed to significantly decrease the occurrence of post-thrombotic syndrome in patients who received this treatment strategy when compared to its occurence in...
The pivotal VeClose trial is investigating the performance of the VenaSeal cyanoacrylate-based adhesive (Medtronic) for vein closure. At LINC 2017, Vascular News caught up with Raghu Kolluri (OhioHealth Vascular Institute, Columbus, USA) who explained his experience with the system...
Primary chronic venous disease is poorly documented in paediatric patients, excluding those diagnosed with Klippel-Trenaunay syndrome (KTS) and post-thrombotic syndrome, writes Dawn Coleman. The Bochum Study previously studied 136 children (aged 10–12 years) longitudinally out to 31 years and...
Having received CE mark approval in July and Health Canada approval in August of last year, FlowAid Medical Technologies has now received US Food and Drug Administration (FDA) clearance for its FA100 SCCD (Sequential Continuous Contraction Device) for the following indications: increase of...
In this clinical study the one-year results of the endovenous laser therapy (ELT) application of 1,940nm laser (Vela XL, Boston Scientific) with respect to feasibility, efficacy and safety are reported. Anna Esipova and colleagues investigated the performance of longer...
The question is often asked: is intravascular ultrasound (IVUS) necessary in a deep venous practice? Stephen Black writes that it was certainly a question that he had asked when initially building his practice. The answer predominantly seemed to be that...
In this interview, Vascular News speaks to Prakash Saha, King’s College Hospital, London, UK, about the growth of deep venous treatment and how the field can best address the ever-growing list of challenges.
What are the challenges you face...
Following Health Canada regulatory approval, BTG is to launch its polidocanol injectable foam (Varithena), a drug/device combination product used to treat varicose veins, in Canada. The product is intended for use in adults with clinically significant venous reflux as...
Two-year outcomes of the VeClose pivotal trial have been reported, showing a 94.3% closure rate and “continued non-inferiority results to radiofrequency ablation” when using the VenaSeal cyanoacrylate embolic adhesive closure system (Medtronic). The data were presented by Raghu Kolluri,...
A Dutch study of greater saphenous vein insufficiency patients has found that mechanochemical ablation (MOCA; ClariVein, Vascular Insights) is an effective and safe treatment with low postoperative pain and fast recovery.
Speaking at the 2016 VEITHsymposium (15–19 November, New...
Fresh data from a Turkish CAPE (cyanoacrylate adhesive perforator embolisation) trial indicate that the method is “as effective as endovenous thermoablative techniques, without the risks of potential inadvertent thermal lesions” for treating incompetent perforating veins. The presentation was given...
Highlights: -Animal experiments raise possibility of wireless vascular flow monitoring -Global variations in abdominal aortic aneurysm care revealed -Jerry Fortuna: Battlefield trauma -Stephen Black: IVUS -Profile: R Clement Darling III
http://venousnews.com/wp-content/uploads/sites/19/2017/01/73-VN-US-v2.pdf
Highlights: -Animal experiments raise possibility of wireless vascular flow monitoring -Global variations in abdominal aortic aneurysm care revealed -Jerry Fortuna: Battlefield trauma -Stephen Black: IVUS -Profile: R Clement Darling III
http://venousnews.com/wp-content/uploads/sites/19/2017/01/73-VN-v2.pdf
Robert Califf is to step down as US Food and Drug Administration (FDA) commissioner upon the inauguration of Donald Trump as US President on 20 January 2017. Democrat Califf, who was appointed to the post in September 2016 by...
The first Hong Kong-based patients diagnosed with pulmonary embolism have been treated using the newly available BTG EKOS system. The EKOS system includes an ultrasonic device that uses acoustic pulses to quickly and safely dissolve blood clots and restore...
Teleflex’ Arrow Vascular positioning system (VPS) Rhythm device with optional TipTracker technology has been issued 510(k) clearance from the US Food and Drug Administration (FDA) to commercialise the device in the USA.
According to a company release, the Arrow VPS...
Abbott has now completed the acquisition of St Jude Medical. The Abbott press release announcing the completion of the acquisition said, “The transaction provides Abbott with expanded opportunities for future growth and is an important part of the company's...
Gerard O’Sullivan, Department of Radiology, University Hospital Galway, Ireland, presented the results of the VIVO-EU prospective study of the Zilver Vena venous stent (Cook Medical) in the treatment of symptomatic iliofemoral outflow obstruction at the Cardiovascular and Interventional Radiological...
The US Food and Drug Administration (FDA) has issued a final rule to ban powdered surgical gloves, patient examination gloves and the absorbable powder used to lubricate surgical gloves.
Citing associations with serious adverse events—including allergic reactions, lung and airway...
The UK’s National Institute for Health and Care Excellence (NICE) has announced its participation in the US Food and Drug Administration (FDA)’s Payer Communication Taskforce. This programme aims to accelerate US patient access to new technologies by gathering evidence...
The first patient has been enrolled in Daiichi Sankyo’s EMIT-AF/VTE (Edoxaban Management in Diagnostic and Therapeutic Procedures) study. This registry will collect real-world clinical data on the use of once-daily edoxaban (Lixiana) with regard to diagnostic and interventional procedures...
LimFlow has received CE mark for its fully percutaneous LimFlow system designed for venous arterialisation of the lower limbs in end-stage patients at risk of limb amputation for critical limb ischaemia.
The LimFlow system is a percutaneous therapy for patients...
Cook Medical has completed enrolment in the first clinical study of an iliofemoral venous stent conducted in the USA under an FDA-approved investigational device exemption. The VIVO clinical study is a prospective, non-randomised, multicentre study intended to evaluate the...
biolitec has announced the development of a “revolutionary, versatile and compact laser for medical application,” with the Leonardo Mini—“a space-saving, efficient, and versatile multi-functional laser,” a company press release says.
The device, weighing only 900g, is available in two designs;...
Highlights: -EVAR 1 trial 15-year follow-up published: Lifelong surveillance of EVAR and prompt reintervention are paramount -IVUS may be a more accurate method than venography for assessing lesion severity and proper venous stent size -Fabio Verzini: EVAR devices -Armando Mansilha:...
Highlights: -EVAR 1 trial 15-year follow-up published: Lifelong surveillance of EVAR and prompt reintervention are paramount -IVUS may be a more accurate method than venography for assessing lesion severity and proper venous stent size -Fabio Verzini: EVAR devices -Armando Mansilha:...
These are exciting times for physicians involved in the management of venous disease. There have been important advances in basic scientific research and crucial developments in venous imaging and diagnostics. Most importantly, however, writes Manj Gohel, physicians now have...
The results of the VIRTUS feasibility study—analysing the performance of the Vici venous stent system (Veniti) in achieving patency of venous lesions up to 12 months—have recently been released, indicating that intravascular ultrasound (IVUS) may be a more accurate...
Jens Eldrup-Jorgensen has been named the new medical director of the Society for Vascular Surgery Patient Safety Organization (SVS PSO). He will replace Jack Cronenwett, who has served as medical director since 2011. The SVS PSO is a part...
The Vascular Society of Great Britain and Ireland celebrates its golden anniversary in Manchester later this month. It was initially founded by Sol Cohen as the Vascular Surgical Society in 1966 with the first two meetings held in London...
Fresh data from the VIRTUS feasibility study presented at the Vascular Interventional Advances (VIVA) annual conference 2016 (18–22 September, Las Vegas, USA) suggest that venography may underestimate the severity of venous lesions and that intravascular ultrasound (IVUS) may be...
The goal of management of venous thromboembolic disease is to prevent thrombus extension or embolisation and to prevent early and late episodes of recurrence. Armando Mansilha, Porto, Portugal, explains how our approach has changed and what obstacles still face...
Janssen Pharmaceuticals and its development partner, Bayer, announced on 23 October 2016 results of two new real-world studies confirming the positive benefit-risk profile of rivaroxaban (Xarelto) in treating venous thromboembolism, and reducing the risk of recurrence.
One study showed that...
Superficial vein thrombosis (SVT) is a common disease affecting 3–11% of the general population, while in patients with varicose veins the incidence of SVT increases to as much as 60%. The prevalence of SVT appears to be two-fold higher...
Lymphoedema is a chronic, progressive condition which can often be debilitating. It is caused by a deficiency or failure of the lymphatic system. Due to its chronic nature, lymphoedema requires ongoing treatment to manage and control the swelling. It...
Ovsep Mandzhikian, Moscow, Russia, reports on his centre’s recent experience of endothermal heat-induced thrombosis following treatment with endovenous laser and radial fibres, suggesting that complications following the treatment are rare and asymptomatic.
Our multicentre observational study summarises the experience of...
Veniti has closed on US$25m in Series D equity financing from Boston Scientific Corporation. The funds will allow Veniti to complete the VIRTUS trial and regulatory filing for the Vici Venous Stent system. The trial is being performed under...
Ash Verma, Clearwater, USA, writes how last year, he happened upon a routine case that struck him as both totally unremarkable and absolutely extraordinary. A 69-year-old female patient presented with one week of swelling and pain in her left...
In this special feature, Vascular News takes a look at occupational radiation—one of the most pressing concerns for vascular and endovascular professionals—speaking to several experts about the dangers of, and strategies to minimise exposure to, radiation in the operating room.
http://vascularnews.com/wp-content/uploads/sites/7/2016/09/Vascular-News-71-Radiation-single-pages.pdf
Highlights: -Iliac branch device results show value of internal iliac artery revascularisation -Absorb bioresorbable scaffold yields 96% primary patency and freedom from reintervention below the knee at 12 months – In the spotlight: Radiation danger -Ragg: Biomatrix sclerofoam -Profile:...
Highlights: -Iliac branch device results show value of internal iliac artery revascularisation -Absorb bioresorbable scaffold yields 96% primary patency and freedom from reintervention below the knee at 12 months - In the spotlight: Radiation danger -Ragg: Biomatrix sclerofoam -Profile:...
Bluegrass Vascular Technologies has received CE mark approval and is launching limited commercial sale of its Surfacer Inside-Out access catheter system. The Surfacer system is indicated for obtaining central venous access to facilitate catheter insertion into the central venous...
A post-market evaluation of the VenaSeal closure system (Medtronic) has found that, at one month, 100% of treated veins remained closed, quality of life scores improved significantly, and return to work and normal activity times were short. The data...
FlowAid Medical Technologies has received CE mark approval for its FA100 SCCD sequential contraction compression device.
According to a press release, the FA100 SCCD was designed with the patient in mind, intended to offer an easy to use system, allowing...
Christopher G Carsten III, Greenville Health System, Greenville, SC, USA
Radiation safety practices have made tremendous advances since the discovery of Roentgen’s X-rays over 120 years ago. The sacrifices of early practitioners have led to the knowledge that now allows...
The US Food and Drug Administration (FDA) has released a draft guidance document entitled “Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices”.
According to an email from the consumer watchdog, the document is intended to clarify “how...
The National Institute for Health and Care Excellence (NICE) has recently issued approval via Interventional Procedure Guidance IPG557 for endovenous mechanochemical ablation for the treatment of varicose veins in the UK. The NICE guidelines provide evidence-based guidance and advice on a range...
BTG has announced that the US Food and Drug Administration (FDA) has approved an extension of the post-activation shelf life of Varithena (polidocanol injectable foam) 1% to 30 days from seven. The announcement was made on 6 July 2016.
“This approval...
6 July 2016
Venous Stenting Training Day
Guys and St Thomas' Hospital, London, UK
http://vascularnews.com/wp-content/uploads/sites/7/2016/06/Final-Flyer-stent-course-SB-2016.pdf
The delivery and exploration of new technology is required to reduce harmful levels of radiation during endovascular procedures, according to Edward Diethrich, a veteran cardiovascular surgeon with first-hand experience of the damage that such exposure can cause.
Following many years’...
Physicians have now treated over 1,000 patients with the Vici Venous Stent. The product received CE mark in October 2013 and was first implanted in January 2014. Subsequently, it has been launched in 13 countries worldwide. Veniti says that...
Therapies in deep venous thrombosis (DVT) have been slow to advance over the past 50 years. Recently, growing awareness and improvements in tools and techniques have piqued interest in venous disease. The ATTRACT trial will try to answer the...
Highlights: -CX acknowledges “huge debt” owed to endovascular pioneers affected by radiation -Audience voting finds a place for false lumen embolisation in chronic type B dissections -Martin Bjӧrck: Iliac artery screening -Ian Franklin: Non-thrombotic iliac lesions -Profile: Wesley Moore
http://vascularnews.com/wp-content/uploads/sites/7/2016/06/70-Vascular-News_US.pdf
Highlights: -CX acknowledges “huge debt” owed to endovascular pioneers affected by radiation -Audience voting finds a place for false lumen embolisation in chronic type B dissections -Martin Bjӧrck: Iliac artery screening -Ian Franklin: Non-thrombotic iliac lesions -Profile: Wesley Moore
http://vascularnews.com/wp-content/uploads/sites/7/2016/06/Vascular-News-70-EU-08.pdf
The US Food and Drug Administration (FDA) has finalised its efforts to streamline the “compassionate use” process, used by physicians to access investigational drugs and biologics for patients with limited treatment options. A statement from Robert Califf, the deputy...
A new bill introduced to the US Senate by Senator Dan Coats seeks to address the gap between the US Food and Drug Administration (FDA)’s priority review process for breakthrough medical devices, and the Centers for Medical and Medicare...
The Netherlands presidency of the European Council and representatives of the European Parliament have reached a political agreement on two draft regulations for medical devices. The new regulations are aimed at ensuring that medical devices and in vitro diagnostic...
At the 2016 Charing Cross Symposium (26–29 April, London, UK) and the International Vein Congress (28–30 April, Miami, USA) Medtronic unveiled clinical data for the VenaSeal closure system demonstrating consistent long-term durability and improved quality of life in patients with venous...
Since its introduction in the late 80s, endovascular therapy has become increasingly widespread and important. A mini-symposium held at the 2016 Charing Cross Symposium (26–29 April, London, UK), brought to the fore the radiation damage that has occurred to...
In a study comparing multiplanar venography to intravascular ultrasound (IVUS) for diagnosing common or external iliac and common femoral vein stenosis, IVUS imaging was found to change both the treatment plan in 60/100 patients and the number of stents...
Two 2016 Charing Cross Symposium (CX 2016; 26–29 April, London, UK) presentations in the Venous Challenges Main Programme session have demonstrated the value of dedicated venous stents, with interim results from two studies in patients with symptomatic iliofemoral venous...
Now that non-thrombotic iliac vein lesions (NIVLs) are more frequently recognised on modern imaging modalities, they are beginning to trigger a paradigm shift in standard vein work whereby there is now increasing emphasis on making sure that these patients...
The first patient suffering from post-thrombotic syndrome associated with venous outflow obstruction has been treated with the VICI VERTO VENOUS STENT system.
This stent delivery system allows the physician to deploy a venous stent beginning at the peripheral end...
A study conducted at St Thomas’ Hospital, London, UK, indicates that the VeinCLEAR catheter (RF Medical) produces good results when used in radiofrequency ablation in the treatment of lower extremity superficial venous insufficiency. The study team, led by Adam...
The Surfacer Inside-Out access catheter system (Bluegrass Vascular Technologies) has been successfully used in its first clinical case. The patient in question was facing life-threatening complications from end-stage renal disease.The procedure was performed under Germany’s "compassionate use" program, which...

“EVRF (F Care Systems) saphenous ablation is a safe and painless procedure for the treatment of the greater saphenous vein and/or small saphenous vein,” according to recently released data. The authors, led by Attila Szabo, VP-Med Health and Education Centre, Budapest, Hungary, write that “high patient acceptance and minimal postoperative discomfort allows a quick return to work and normal life for patients.”
EVRF is a monopolar radiofrequency ablation system for endothermal treatment of small, midsize and large veins. “Endovenous procedures are far less invasive than surgery and have lower complication rates,” the authors write. “The procedure is well tolerated by the patients, and it produces good cosmetic results. Excellent clinical results are seen at four to five years, and the long-term efficacy of the procedure in now known with 10 years of experience.”
Szabo and colleagues evaluated the effectiveness of EVRF treatment and analysed the three-year results of using the device for endovenous radiofrequency ablation and spider vein treatments from July 2011 to March 2015 to treat 751 saphenous reflux and varicosity patients.
Radiofrequency ablation using the EVRF CR45i catheter was performed on 751 limbs (628 greater saphenous vein, 102 small saphenous vein and 21 for both). Complete occlusion was found in 743 of 751 cases (99%) at one month. At one-year follow-up, 15 of 530 patients showed recanalisation longer than 5cm (97.2% one-year occlusion) without clinical symptoms. The three-year occlusion rate was 96.6% (two of 59 patients had an open vein segment). At one-year follow-up the investigators evaluated the postoperative pain reported by the patients on a visual analogue scale (2.4 preoperatively, 1.2, 0.4 and 0.1 at one week, one month and one year postoperatively, respectively) and Venous Clinical Severity Scores (7.7, 3.9 and 1.8 before surgery, at one month and at one year, respectively). The average patient satisfaction was 99% at one year. There were no cases of deep vein thrombosis, skin burns, neuritis or bleeding. “Minimal” bruising was observed at the treatment site of the tributaries in some cases and four patients had mild inflammation which was treated conventionally.
For smaller veins, more than 5,000 treatment sessions for teleangiectasias and spider veins on the face and lower limbs were completed. The authors used radiofrequency treatment or combined the treatment with liquid sclerotherapy. Facial recovery rate was 100% and lower limb recovery rate was 82% on average. The treatments were “well tolerated—although the pain level always depends on the patients’ current psychic status—and the patient satisfaction rate was high,” the authors report.
Szabo and colleagues suggest that “EVRF is appropriate for the treatment of teleangiectasias, reticular veins with the hand piece and K3i or K6i needles (face or lower leg), small varicose veins or tributaries 2–5mm with the hand catheters and for saphenous truncs and perforators 4–15mm in diameter with the CR45i catheter.”
“The procedure, under local tumescent anaesthesia, is simple and the disposable devices are easy to use. The EVRF hand piece with K3i and K6i needles proved to be useful in teleangiectasia treatment, with excellent results,” Szabo et al conclude. “This radiofrequency procedure is also suitable for combination with liquid or foam sclerotherapy.”
Boehringer Ingelheim has announced the enrolment of the first patient in RE-COVERY DVT/PE, a global observational study on the management of deep vein thrombosis (DVT) and pulmonary embolism (PE).
“Large pivotal randomised trials have revolutionised our strategies to manage and...
The US Food and Drug Administration has announced a proposal to ban most powdered gloves in the USA. While use of these gloves is decreasing, they pose an unreasonable and substantial risk of illness or injury to health care...
Highlights: -Tackling the challenges in the vascular and endovascular arena -CX Venous Workshop -CX Vascular Access course -CX Edited Live Cases -CX ilegx Collaboration Day -CX Acute Stroke Challenges Programme -Summary of Vascular News and Interventional News profiles 2015
http://vascularnews.com/wp-content/uploads/sites/7/2016/03/VNCharingCross2016US_lowres.pdf
Highlights: -Tackling the challenges in the vascular and endovascular arena -CX Venous Workshop -CX Vascular Access course -CX Edited Live Cases -CX ilegx Collaboration Day -CX Acute Stroke Challenges Programme -Summary of Vascular News and Interventional News profiles 2015
http://vascularnews.com/wp-content/uploads/sites/7/2016/03/VNCharingCross2016EU_lowres.pdf
Hybrid operative thrombectomy achieves complete thrombus resolution in one operating room trip which results in reduced length of stay, bleeding complications and transfusions when compared with percutaneous treatments that use thrombolytic therapy. This can be achieved without sacrificing successful...
A few months ago a discussion on the LinkedIn page of the American College of Phlebology caught my attention: “Medicare: maximum four endovenous laser
therapies per lifetime?”
In Belgium we can use one radiofrequency catheter or endovenous laser ablation fibre...
According to the World Health Organization, around 300 million people are affected by the pathological oedema of limbs. Lymphoedema is caused by partial or total obstruction of lymphatic collectors as a consequence of skin and deep soft tissue inflammation,...
Saphenous pulsation is the cyclical impulse of antegrade flow observed on colour duplex ultrasound after prolonged standing. It is a common observation in patients with saphenous reflux and is detected in the upper part of the great saphenous vein,...
AngioDynamics has signed an agreement with Merz North America, a US affiliate of the global Merz Pharma Group, to serve as the exclusive distributor of Asclera (polidocanol) injection within the US vein market.
Asclera is approved for the treatment of uncomplicated spider and uncomplicated...
At the 2016 Leipzig Interventional Course meeting (LINC; 26–29 January, Leipzig, Germany), Michael R Jaff, Boston, USA, discussed the challenges facing intervention for deep vein thrombosis and pulmonary embolism, and when using an interventional approach may be preferable.
“When you...
The US Food and Drug Administration (FDA) has confirmed the appointment of Robert Califf as its 22nd commissioner, following a US Senate vote of 88 to 4 in his favour.
Califf was nominated to replace previous commissioner, Margeret A Hamburg, by...
ArtVentive Medical has announced enrolment in the ongoing OCCLUDE post-market surveillance study. This study aims to further the use of the EOS endoluminal occlusion system for the treatment of venous and arterial cases in which precise placement and immediate,...
Highlights: -Neuromonitoring changes trigger protocol against spinal cord ischaemia during TEVAR -New European industry code: Sponsorship of physicians to attend congresses to be done via healthcare organisations -In the spotlight: Atherectomy -Raphaël Coscas: Vascular access -Profile: Jonathan Beard
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/69-Vascular-News_US.pdf
The kit comprises the company's DMX Doppler, probes and accessories to aid the vascular, diabetic or lymphatic specialist with the assessment of arterial disease and neuropathy.
Cook Medical has initiated a voluntary recall of 360 specific lots of single lumen central venous catheters and pressure monitoring sets and trays due to catheter tip fracture and/or separation. Globally, 17,827 devices are subject to this recall.
Huntleigh has launched the Dopplex DMX digital vascular Doppler. According to a company release, this Doppler combines audio clarity and a visual representation of waveforms with high performance probe sensitivity.
Highlights: -Neuromonitoring changes trigger protocol against spinal cord ischaemia during TEVAR -New European industry code: Sponsorship of physicians to attend congresses to be done via healthcare organisations -In the spotlight: Atherectomy -Raphaël Coscas: Vascular access -Profile: Jonathan Beard
http://vascularnews.com/wp-content/uploads/sites/7/2016/02/69-Vascular-News_EU.pdf
Rick De Graaf, Department of Radiology, Maastricht University Medical Centre, Maastricht, The Netherlands, presented the results of the first comparative study of four dedicated stents for the venous system at the VEITHsymposium (17–20 November 2015, New York, USA). De...
Researchers have activated the first 10 medical sites and enrolled the first nine patients in a study that will determine the safety and effectiveness of inferior vena cava filters, small, cage-like devices implanted to prevent life-threatening blood clots from...
Bristol-Myers Squibb and Pfizer have announced results from a post-hoc early time course subanalysis of the phase 3 AMPLIFY (Apixaban for the initial management of pulmonary embolism and deep vein thrombosis as first-line therapy) trial.
The subanalysis demonstrated Eliquis (apixaban) was comparable to...
Bayer and its development partner Janssen Pharmaceuticals presented results from two real world studies—the non-interventional XALIA study in patients with deep vein thrombosis (DVT) and a study looking at patients with cancer-associated thrombosis on 8 December 2015. Results from...
The European Diagnostics Manufacturers Association (EDMA) and the European Medical Technology Industry (Eucomed) have approved a new joint code of conduct that stipulates that, after 31 December 2017, industry should no longer provide direct “financial or in kind support”...
Highlights: -IN.PACT SFA two-year results "have potential to drive paradigm shift" in femoropopliteal lesion treatment -PERICLES study helps bring chimney technique "out of the shadows" -Endologix and TriVascular announce merger -Jeffrey P Carpenter: Protection devices -Peter H Lin: Thrombolysis...
Highlights: -IN.PACT SFA two-year results "have potential to drive paradigm shift" in femoropopliteal lesion treatment -PERICLES study helps bring chimney technique "out of the shadows" -Endologix and TriVascular announce merger -Jeffrey P Carpenter: Protection devices -Peter H Lin: Thrombolysis...
By Athanasios D Giannoukas
Today, venous stenting plays an important role in the treatment of deep venous pathologies. The current indications for its use include acute iliofemoral thrombosis after catheter-directed or pharmacomechanical thrombolysis to resolve residual iliac stenosis, May-Thurner syndrome and...
According to new a study published in PLOS Medicine, a new clinical model can help predict the risk of venous thromboembolism (VTE) among patients with a leg case, enabling doctors to identify high risk cases.
Using data from three large cohorts,...
Medtronic’s VenaSeal closure system has been granted pre-market approval (PMA) from the US Food and Drug Administration (FDA) for the treatment of symptomatic venous reflux.
This minimally invasive procedure is the first and only non-tumescent, non-thermal, non-sclerosant procedure approved for...
Peter H Lin
Pulmonary embolism in children is a potentially lethal condition and yet is a vastly understudied arena. Autopsy studies show a higher prevalence of pulmonary embolism compared to medical database registries suggesting that this condition is often clinically...
Highlights: -"Working with radiation is like keeping a pet tiger in your living room" -Wearable exercise tracker improves intermittent claudication symptoms at six months -Endovascular revolution in the aorta: 25 years of a landmark case -Profile: Sebastian Debus -Rabih...
Highlights: -"Working with radiation is like keeping a pet tiger in your living room" -Wearable exercise tracker improves intermittent claudication symptoms at six months -Endovascular revolution in the aorta: 25 years of a landmark case -Profile: Sebastian Debus -Rabih...
At procedure, Zilver Vena stent placement resulted in a more than two-fold improvement in the vessel minimum lumen diameter. Based on available follow-up data, stent placement has corresponded to improved clinical symptoms.
The City University of New York (CUNY), Lawrence Livermore National Laboratory, and Near Infrared Imaging, have released the “Vein-Eye” camera. The Vein-Eye provides enhanced visualisation of veins when drawing blood or placing IVs in a patient’s arm or hand....
By Johann Chris Ragg
Patients with saphenous insufficiency can undergo valve zone shaping instead of destructive methods by surgical removal or endovenous closure, says Johann Chris Ragg. He presented the new vein restoring modality at the European Venous Forum (EVF;...